Functional Materials for LIB 锂离子电池材料.docx
《Functional Materials for LIB 锂离子电池材料.docx》由会员分享,可在线阅读,更多相关《Functional Materials for LIB 锂离子电池材料.docx(9页珍藏版)》请在冰豆网上搜索。
FunctionalMaterialsforLIB锂离子电池材料
Report
FunctionalMaterialsforLithium-IonBatteries
1.Introduction
Energyisoneofthemostimportanttopicsinthe21stcentury.Withtherapiddepletionoffossilfuelsandincreasinglyworsenedenvironmentalpollutioncausedbyvastfossil-fuelconsumption,thereishighdemandtomakeefficientuseofenergyandtoseekrenewableandcleanenergysourcesthatcansubstitutefossilfuelstoenablethesustainabledevelopmentofoureconomyandsociety.Energystorage,anintermediatesteptotheversatile,clean,andefficientuseofenergy,hasreceivedworldwideconcernandincreasingresearchinterest.
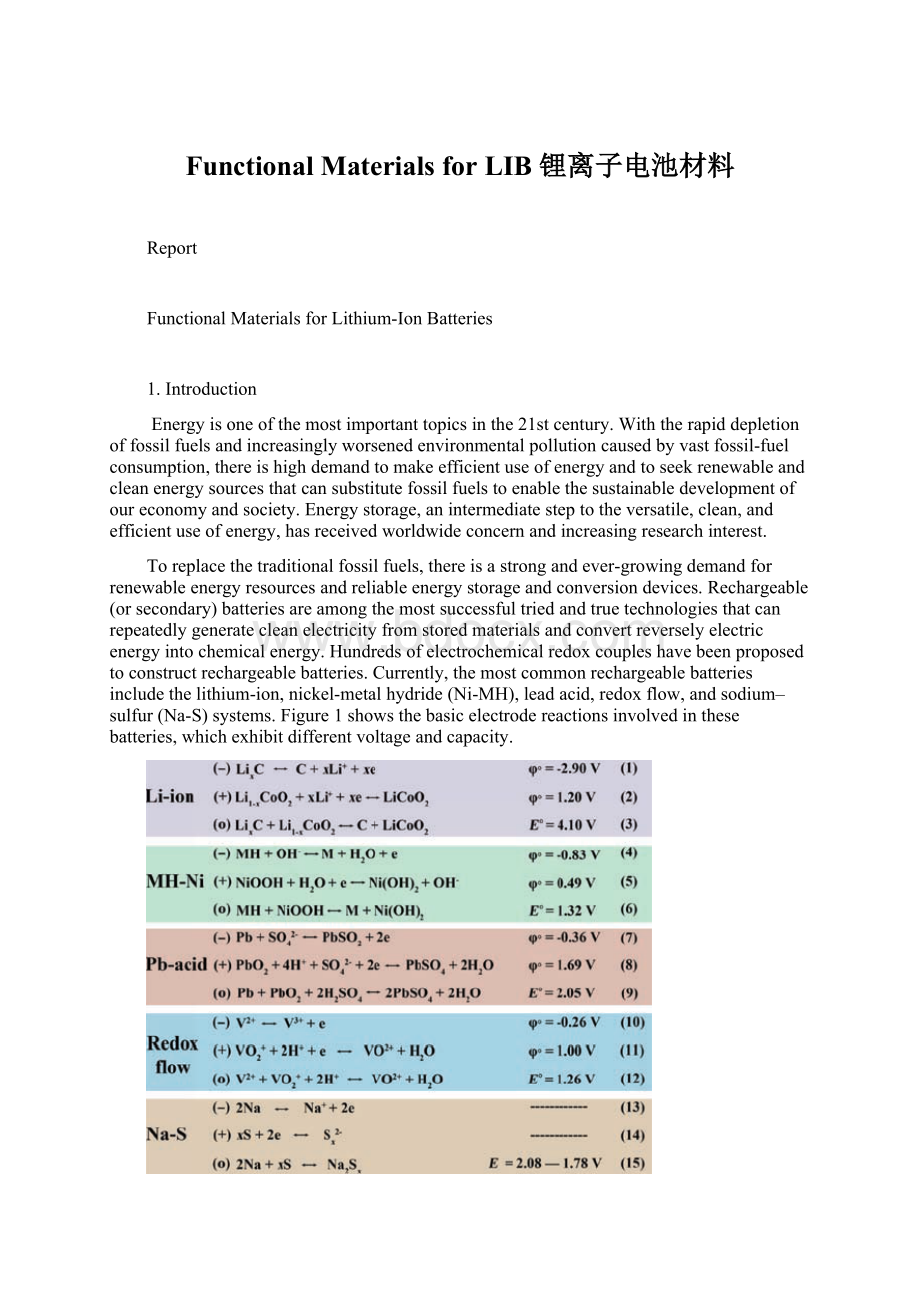
Toreplacethetraditionalfossilfuels,thereisastrongandever-growingdemandforrenewableenergyresourcesandreliableenergystorageandconversiondevices.Rechargeable(orsecondary)batteriesareamongthemostsuccessfultriedandtruetechnologiesthatcanrepeatedlygeneratecleanelectricityfromstoredmaterialsandconvertreverselyelectricenergyintochemicalenergy.Hundredsofelectrochemicalredoxcoupleshavebeenproposedtoconstructrechargeablebatteries.Currently,themostcommonrechargeablebatteriesincludethelithium-ion,nickel-metalhydride(Ni-MH),leadacid,redoxflow,andsodium–sulfur(Na-S)systems.Figure1showsthebasicelectrodereactionsinvolvedinthesebatteries,whichexhibitdifferentvoltageandcapacity.
Figure1.Materialchemistryofrepresentativerechargeablebatteries.ΦoandEoarethestandardreductionpotentialofthehalfcellandtheoverallcellat25℃,respectively.FortheNa-Ssystem,theelectrodepotentialvarieswithoperatingtemperature.
Undoubtedly,lithium-ionbatteriesarethemostpopularrechargeablebatteries.Duringthepastthreedecades,tremendousresearchefforthasbeendevotedtoinvestigatingtheelectrochemicalperformanceofawidevarietyofactivematerialsandelectrolytes,asoutlinedinFigure2.
Figure2.Representativeapplicationsforrechargeablebatteries.
SinceresearchersatSonyEnergytechdevelopedthefirstcommercialLi-ionbatteriesinthelate1980s,avarietyofeffortshavebeenundertakentoimprovethebatterymaterials.ALIBismainlycomposedofananode(negative),acathode(positive),anelectrolyte,andaseparator.ThepositiveelectrodematerialsaretypicallyLi-containingmetaloxideswithalayeredstructure(suchaslithiumcobaltoxide)ortunnel-structuredmaterials(suchaslithiummanganeseoxide).Thenegativeelectrodematerialsincludeinsertion-typematerials(suchascarbon,Li4Ti5O12,TiO2,etc.),conversion-typematerials(suchasironoxides,nickeloxides,cobaltoxides,etc.),andalloying-typematerials(suchasSi,Sn,etc.).Theelectrolyteshouldbeagoodionicconductorandelectronicinsulator.Mostoftheelectrolytesarebasedonthesolutionofinorganiclithiumsaltsdissolvedinamixtureoftwoormoreorganicsolvents.ThefunctionoftheseparatoristopreventshortcircuitingbetweenthenegativeandpositiveelectrodesandtoprovideabundantchannelsfortransportationofLiionduringcharging/discharging.Whenabatteryiscycled,Liionsexchangebetweenthepositiveandnegativeelectrodes.Hereinthisreport,areviewofthelatestprogressisprovided,withamainfocusonthenewcathodeandanodematerialsandnovelelectrodestructures,suchasnanostructuresandnanocomposites.
2.AnodeMaterials
2.1Carbonaceousmaterials
CommercialLIBsareusuallybasedoncarbonaceousanodematerials,inwhichLiisinsertedduringcharging.TheresultingLi-interactedcarbonsshowalowpotentialclosetothatofametallicLielectrode.Byusingacarbonaceousanode,theknottyproblemofdendriteformationintheinitiallyemployedLimetalanodecanbeavoided,andthesafetyandreliabilityofLIBsareimproved.Carbonmaterialscanbetypicallyspecifiedintothreegroups,namely,graphiteandgraphitizedmaterials,ungraphitizedsoftcarbon,andhardcarbon.Graphiteismostwidelyusedduetoitsstablespecificcapacity,smallirreversiblecapacity,andgoodcyclingperformance.Intercalation/deintercalationofLi+inandoutofthegraphiteuponcharge/dischargeformsthebasisofgraphiteastheanodeofthebattery(Figure3).Mesocarbonmicrobeads(MCMBs)derivedfrompetroleumorcoalproductsexhibitdesirableoverallperformancewithmoderatehighcapacityandexcellentcyclability.Theelectrochemicalpropertiesofsoftcarbonsheat-treatedbelow1000℃andhardcarbonsarelargelydeterminedbytheirsurfacecharacteristicsandporestructures.Hardcarbondisplaysahighlithiumstoragecapacityandgoodpowercapability,butpoorelectricalconductivityandalargeirreversiblecapacity.Althoughcarbonousanodematerialsreceivewide-rangeapplicationsincommercializedLIBs,itisrecognizedthatgraphiticcarbonssufferfromsolventco-intercalationinpropylene-carbonate-basedelectrolytes,whichresultsinlargeinterlayerexpansionandsubsequentdegradationofthegraphiticstructure.Moreimportantly,thegravimetricandvolumetriccapacityofcarbonmaterialsislimited.TherapiddevelopmentofelectronicdevicesandEVsdemandsamuchhigherenergydensityaswellasahigherpowerdensityandasmallerirreversiblecapacityforanodes.
Figure3.SchematicrepresentationoftheworkingprincipleaLi-ionbattery.
2.2Lithiumalloys
Somemain-groupelements(e.g.,Si,Ge,Sn,Al,Bi,Zn,andSb)canalloywithlithiumatalowpotential.Representatively,SiandSncangeneratelithiumalloyswithLicompositionsuptoLi4.4M,givingtheoreticalspecificcapacitiesashighas4200and993mAh/g,respectively.Theirvolumetriccapacitiesalsoexceedthatofgraphite(1750mAh/cm3forSiand1000mAh/cm3forSnversus855mAh/cm3forgraphite).Figure4comparesthetheoreticalgravimetricandvolumetriccapacitiesofLixMalloysatfulllithiationandthecapacityfortheintercalationoflithiumintocarbon.Unfortunately,hugevolumechangesoccurduringtheelectrochemicallithiation/delithiationprocess.Forexample,avolumeexpansionontheorderof400%occurredduringtheformationofLi4.4Sialloy,whichcausescrackingandeventualpulverizationoftheelectrodeandresultsinrapidcapacitydecline.Anotherdisadvantageassociatedwiththesiliconanodeisthepoorelectronicconductivity,whichimpedesfastlithiation.Toovercomethesehurdles,substantialefforthasbeenfocusedonusingnanoscalematerialsand/ordispersingtheactivecomponentinarigidmatrixtoformcompositestructures.
Figure4.Thetheoreticalspecificandvolumetriccapacitiesofvariousfullylithiatedphasesofelectrochemicallyactivemetalelements.Thevolumetriccapacityiscalculatedusingthefullylithiatedvolume.
Nanostructuredalloysaremoreflexibletoaccommodatethestraininducedbyvolumevariationsbecausethetoughnessandadhesioneffectwithingrainboundariesmayincreaseatthenanoscale.Thedurabilityofnanoparticlesstemsfromtwoaspects.First,thesmallsizelimitsthemaximumpossiblelithiumconcentrationgradient.Moreover,thesurfaceisunconstrainedinthenormaldirectionandthusprovidesstressreductioninthesurfacevicinity,whichencompassessmostofthegrainsfornanoparticles.
Somepeculiarlydesignednanoscaleelectrodes,suchashollownanostructures,havemorefreespaceandcanendurelargerstresses.Forexample,nest-likeSihollownanospheresallowedfastionicdiffusionandfacilestrainrelaxation,yieldingasignificantlyimprovedreversiblecapacityandrateperformanceincomparisonwiththeirbulkcounterparts.Inaddition,aSinanowirearrayfirmlygrownonacurrentcollectorenabledefficient1Delectrontransportalongeachwirewhileundergoingsuperplasticdeformationuponalloyinganddealloying.Inthecaseoftin-basedmaterials,porousstructuresincludingSn@Cnanoparticlesencapsulatedinbamboo-likehollowcarbonnanofibers,Snnanoparticlesconfinedinelastichollowcarbonspheres,SnO2nanoparticlesloadingoncross-stackedcarbonnanotubesheets,andcoaxialSnO2@Chollowspheresprovedtobeinterestinganodecandidatesforhighlyreversiblelithiumstorage.
Anotherinnovativeapproachisemployingintermetallicphases,MM′,whereMistheactiveelementthatcanelectrochemicallyalloywithLiandM′isaninactivemetal(e.g.,Cu,Ni,Co,andFe)thatsuppressesthevolumechange.Asanexample,Ni3Sn4nanoparticlesdepositedoncoppernanorodscouldmaintainhighcapacityforhundredsofcycles.Interestingly,theelectrodemorphologywasessentiallypreservedaftercycling,incontrasttotheformationofcracksonthesurfaceofcompactelectrodes.Theexcellentcapacityretentionwaslargelyattributedtothelargefreespacebetweenthealloypillarsthateffectivelyaccommodatedthevolumevariation.
2.3Transitionmetaloxides
In2000,Poizotetal.reportedforthefirsttimethatlithiumcanbestoredreversiblyintransitionmetal(TM)oxidesthroughaheterogeneousconversionreaction:
Li+TMO→Li2O+TM
whereTMisCo,Fe,Ni,andCu.Later,reversiblelithiumstoragewasalsoobservedinTMfluorides,sulfides,nitrides,andphosphides.ItisveryinterestinginviewoffundamentalresearchthatveryinertLiForLi2OcanreactwithaTMatroomtemperature.Thiswasunexpectedpreviously.
Thereversibleelectrochemicallithiumstorageproceedsmoreeasilywiththenanoscaleelectrode.Therefore,researchontransition-metaloxideshasfocusedonnanostructures.SimilartotheLi-alloyingprocess,theconversionreactionleadstovolumevariationupontheelectrochemicalcycling.Conceptually,efficientapproachessuchasreducingparticlesizeorusingnanocompositesareapplicabletometal-oxidebasedanodematerialsaswell.
Apartfromtheprom