专业英语教学.docx
《专业英语教学.docx》由会员分享,可在线阅读,更多相关《专业英语教学.docx(35页珍藏版)》请在冰豆网上搜索。
专业英语教学
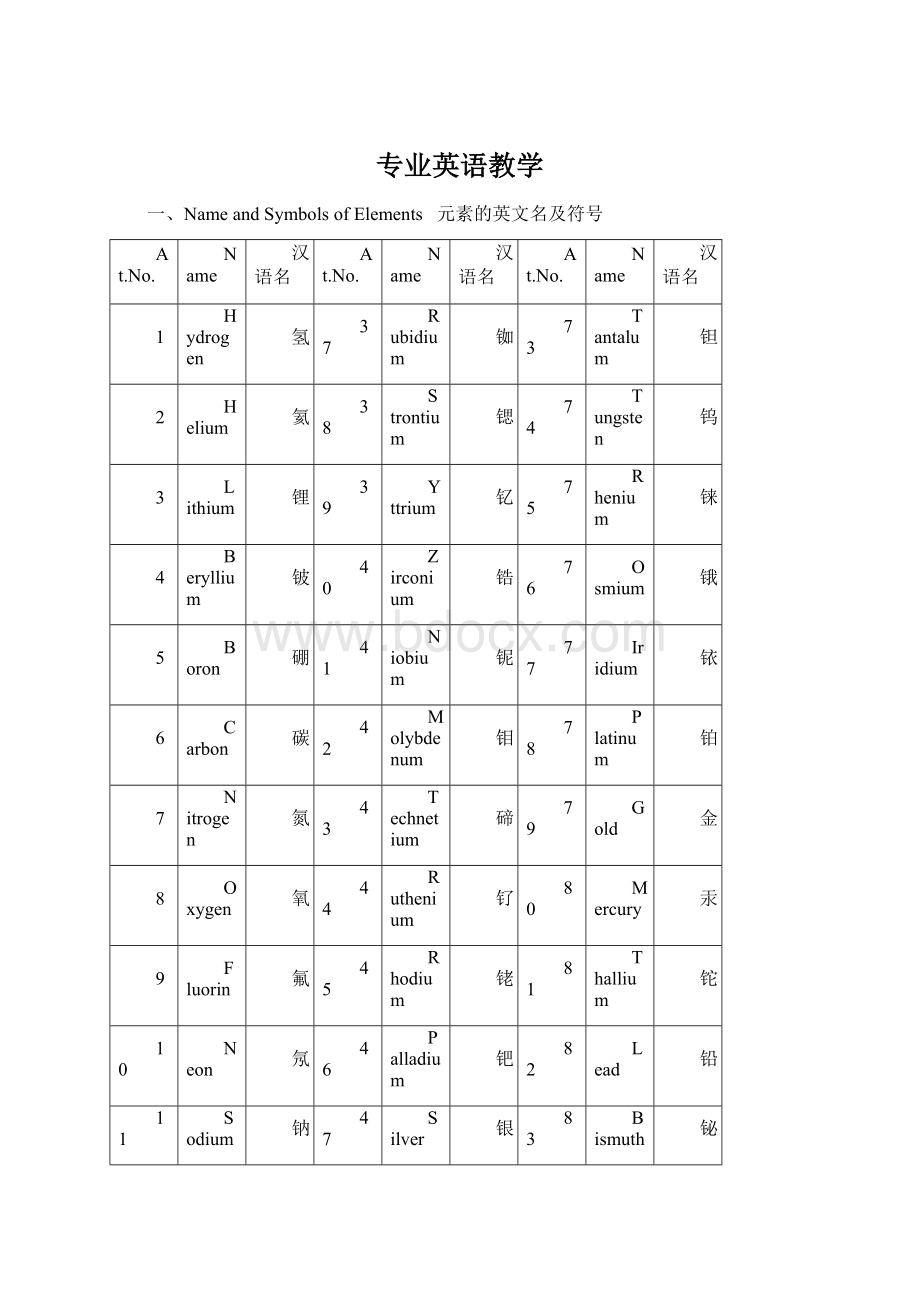
一、NameandSymbolsofElements 元素的英文名及符号
At.No.
Name
汉语名
At.No.
Name
汉语名
At.No.
Name
汉语名
1
Hydrogen
氢
37
Rubidium
铷
73
Tantalum
钽
2
Helium
氦
38
Strontium
锶
74
Tungsten
钨
3
Lithium
锂
39
Yttrium
钇
75
Rhenium
铼
4
Beryllium
铍
40
Zirconium
锆
76
Osmium
锇
5
Boron
硼
41
Niobium
铌
77
Iridium
铱
6
Carbon
碳
42
Molybdenum
钼
78
Platinum
铂
7
Nitrogen
氮
43
Technetium
碲
79
Gold
金
8
Oxygen
氧
44
Ruthenium
钌
80
Mercury
汞
9
Fluorin
氟
45
Rhodium
铑
81
Thallium
铊
10
Neon
氖
46
Palladium
钯
82
Lead
铅
11
Sodium
钠
47
Silver
银
83
Bismuth
铋
12
Magnesium
镁
48
Cadmium
镉
84
Polonium
钋
13
Aluminium
铝
49
Indium
铟
85
Astatine
砹
14
Silicon
硅
50
Tin
锡
86
Radon
氡
15
Phosphorus
磷
51
Antimony
锑
87
Francium
钫
16
Sulfur
硫
52
Tellurium
锝
88
Radium
镭
17
Chlorine
氯
53
Iodine
碘
89
Actinium
锕
18
Argon
氩
54
Xenon
氙
90
Thorium
钍
19
Potassium
钾
55
Cesium
铯
91
Protactinium
镤
20
Calcium
钙
56
Barium
钡
92
Uranium
铀
21
Scandium
钪
57
Lanthanum
镧
93
Neptunium
镎
22
Titanium
钛
58
Cerium
铈
94
Plutonium
钚
23
Vanadium
钒
59
Praseodymium
镨
95
Americium
镅
24
Chromium
铬
60
Neodymium
钕
96
Curium
锔
25
Manganese
锰
61
Promethium
钷
97
Berkelium
锫
26
Iron
铁
62
Samarium
钐
98
Californium
锎
27
Cobalt
钴
63
Europium
铕
99
Einsteinium
锿
28
Nickel
镍
64
Gadolinium
钆
100
Fermium
镄
29
Copper
铜
65
Terbium
铽
101
Mendelevium
钔
30
Zinc
锌
66
Dysprosium
镝
102
Nobelium
锘
31
Gallium
镓
67
Holmium
钬
103
Lawrencium
铹
32
Germanium
锗
68
Erbium
铒
104
Rutherfordium
33
Arsenic
砷
69
Thulium
铥
105
Dubnium
34
Selenium
硒
70
Ytterbium
镱
106
Seaborgium
35
Bromine
溴
71
Lutetium
镥
107
Bohrium
36
Krypton
氪
72
Hafnium
铪
108
Hassium
109
Meitnerium
二、IUPACnomenclatureofinorganicchemistry无机物的命名
TheIUPACnomenclatureofinorganicchemistryisasystematicmethodofnaminginorganicchemicalcompoundsasrecommendedbytheInternationalUnionofPureandAppliedChemistry(IUPAC).Ideally,everyinorganiccompoundshouldhaveanamefromwhichanunambiguous(意思清楚的;明确的;毫不含糊的;无歧义的)formulacanbedetermined.ThereisalsoanIUPACnomenclatureoforganicchemistry.
Thenames"caffeine"and"3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione"bothsignifythesamechemical.Thesystematicnameencodesthestructureandcompositionofthecaffeinemoleculeinsomedetail,andprovidesanunambiguousreferencetothiscompound,whereasthename"caffeine"justnamesit.Theseadvantagesmakethesystematicnamefarsuperiortothecommonnamewhenabsoluteclarityandprecisionarerequired.However,forthesakeofbrevity,evenprofessionalchemistswillusethenon-systematicnamealmostallofthetime,becausecaffeineisawell-knowncommonchemicalwithauniquestructure.Similarly,H2OismostoftensimplycalledwaterinEnglish,thoughotherchemicalnamesdoexist.
1.Singleatomanionsarenamedwithan-idesuffix:
forexample,H−ishydride.
2.Compoundswithapositiveion(cation),thenameofthecompoundissimplythecation'sname(usuallythesameastheelement's),followedbytheanion.Forexample,NaClissodiumchloride,andCaF2iscalciumfluoride.
3.CationsabletotakeonmorethanonepositivechargearelabeledwithRomannumeralsinparentheses.Forexample,Cu+iscopper(I),Cu2+iscopper(II).Anolder,deprecatednotation(推荐使用符号)istoappend-ousor-ictotherootoftheLatinnametonameionswithalesserorgreatercharge.Underthisnamingconvention,Cu+iscuprousandCu2+iscupric.Fornamingmetalcomplexesseethepageoncomplex(chemistry).
4.Oxyanions(polyatomicanionscontainingoxygen,含氧阴离子)arenamedwith-iteor-ate,foralesserorgreaterquantityofoxygen.Forexample,NO2−isnitrite,whileNO3−isnitrate.Iffouroxyanionsarepossible,theprefixeshypo-andper-areused:
hypochloriteisClO−,perchlorateisClO4−,
5.Theprefixbi-isadeprecated(推荐使用)wayofindicatingthepresenceofasinglehydrogenion,asin"sodiumbicarbonate"(NaHCO3).Themodernmethodspecificallynamesthehydrogenatom.Thus,NaHCO3wouldbepronounced"sodiumhydrogencarbonate".
Positivelychargedionsarecalledcationsandnegativelychargedionsarecalledanions.Thecationisalwaysnamedfirst.Ionscanbemetalsorpolyatomicions.Thereforethenameofthemetalorpositivepolyatomicionisfollowedbythenameofthenon-metalornegativepolyatomicion.Thepositiveionretainsitselementnamewhereasforasinglenon-metalaniontheendingischangedto-ide.
Example:
sodiumchloride,potassiumoxide,orcalciumcarbonate.
Whenthemetalhasmorethanonepossibleionicchargeoroxidationnumberthenamebecomesambiguous.InthesecasestheoxidationnumberofthemetalionisrepresentedbyaRomannumeralinparenthesesimmediatelyfollowingthemetalionname.Forexampleinuranium(VI)fluoridetheoxidationnumberofuraniumis6.Anotherexampleistheironoxides.FeOisiron(II)oxideandFe2O3isiron(III)oxide.
Anoldersystemusedprefixesandsuffixestoindicatetheoxidationnumber,accordingtothefollowingscheme:
Oxidationstate
Cationsandacids
Anions
Lowest
hypo--ous
hypo--ite
-ous
-ite
-ic
-ate
Highest
per--ic
per--ate
Thusthefouroxyacidsofchlorinearecalledhypochlorousacid(HOCl),chlorousacid(HOClO,亚氯酸),chloricacid(HOClO2)andperchloricacid(HOClO3),andtheirrespectiveconjugatebasesarethehypochlorite,chlorite,chlorateandperchlorateions.Thissystemhaspartiallyfallenoutofuse,butsurvivesinthecommonnamesofmanychemicalcompounds:
themodernliteraturecontainsfewreferencesto"ferricchloride"(insteadcallingit"iron(III)chloride"),butnameslike"potassiumpermanganate"(insteadof"potassiummanganate(VII)")and"sulfuricacid"abound.
Contents
∙1Traditionalnaming
o1.1Namingsimpleioniccompounds
▪1.1.1Listofcommonionnames
o1.2Naminghydrates
o1.3Namingmolecularcompounds
o1.4Namingacids
∙22005revisionofIUPAC'snomenclatureforinorganiccompounds
∙3Seealso
∙4References
∙5Externallinks
Traditionalnaming
Namingsimpleioniccompounds
Anioniccompoundisnamedbyitscationfollowedbyitsanion.Seepolyatomicionsforalistofpossibleions.
Forcationsthattakeonmultiplecharges,thechargeiswrittenusingRomannumeralsinparenthesesimmediatelyfollowingtheelementname)Forexample,Cu(NO3)2iscopper(II)nitrate,becausethechargeoftwonitrateions(NO3-1)is2×−1=−2,andsincethenetchargeoftheioniccompoundmustbezero,theCuionhasa2+charge.Thiscompoundisthereforecopper(II)nitrate.Inthecaseofcationswitha4+oxidationstate,theacceptableformatfortheRomannumeral4isIVandnotIIII.
TheRomannumeralsinfactshowtheoxidationnumber,butinsimpleioniccompounds(i.e.,notmetalcomplexes)thiswillalwaysequaltheionicchargeonthemetal.Forasimpleoverviewsee[1],formoredetailsseeselectedpagesfromIUPACrulesfornaminginorganiccompounds.
Listofcommonionnames
Monatomicanions:
Cl−chloride
S2−sulfide
P3−phosphide
Polyatomicions:
NH4+ammonium
H3O+hydr-oxonium
NO3−nitrate
NO2−nitrite
ClO−hypochlorite
ClO2−chlorite
ClO3−chlorate
ClO4−perchlorate
SO32−sulfite
SO42−sulfate
HSO3−hydrogensulfite(orbisulfite)
HCO3−hydrogencarbonate(orbicarbonate)
CO32−carbonate
PO43−phosphate
HPO42−hydrogenphosphate
H2PO4−dihydrogenphosphate
CrO42−chromate
Cr2O72−dichromate
BO33−borate
AsO43−arsenate
C2O42−oxalate
CN−cyanide
SCN−thiocyanate
MnO4−permanganate
Naminghydrates(水合物)
Hydratesareioniccompoundsthathaveabsorbedwater.Theyarenamedastheioniccompoundfollowedbyanumericalprefixand-hydrate.Thenumericalprefixesusedarelistedbelow:
1.mono-
2.di-
3.tri-
4.tetra-
5.penta-
6.hexa-
7.hepta-
8.octa-
9.nona-
10.deca-
Forexample,CuSO4·5H2Ois"copper(II)sulfatepentahydrate".
Namingmolecularcompounds
Inorganicmolecularcompoundsarenamedwithaprefix(seelistabove)beforeeachelement.Themoreelectronegativeelementiswrittenlastandwithan-idesuffix.Forexample,CO2iscarbondioxide.AlthoughCCl4issometimescalledcarbontetrachlorideunderthisrule,itisnotaninorganicmoleculeandismoreproperlycalledtetrachloromethane.Therearesomeexceptionstotherule,however.Theprefixmono-isnotusedwiththefirstelement;forexample,CO2iscarbondioxide,not"monocarbondioxide".Sometimesprefixesareshortenedwhentheendingvowel(元音)oftheprefix"conflicts"withastartingvowelinthecompound.Thismakesthecompoundeasiertospeak;forexample,COis"carbonmonoxide"(asopposedto"monooxide").
Namingacids
Acidsarenamedbytheaniontheyformwhendissolvedinwater.Ifanacidformsananionnamed___ide,itisnamedhydro___icacid.Forexample,hydrochloricacidformsachlorideanion.Withsulfur,however,thewholewordiskeptinsteadoftheroot:
i.e.:
hydrosulfuricacid.Secondly,anionswithan-atesuffixareformedwhenacidswithan-icsuffixaredissolved,e.g.chloricacid(HClO3)dissociatesintochlorateanionstoformsaltssuchassodiumchlorate(NaClO3);anionswithan-itesuffixareformedwhenacidswithan-oussuffixaredissolvedinwater,e.g.chlorousacid(HClO2)disassociatesintochloriteanionstoformsaltssuchassodiumchlorite(NaClO2).
三、LabApparatusName实验室基本仪器名称
大口杯/烧杯beaker
玻璃烧杯glassbeaker
聚四氟乙烯烧杯PTFEgriffinbeaker
塑料烧杯plasticbeaker
不锈钢杯stainless-steelbeaker
玻璃瓶flask
三角瓶co