鼻腔和口腔吸入制剂指导原则-EMA(翻译版).pdf
《鼻腔和口腔吸入制剂指导原则-EMA(翻译版).pdf》由会员分享,可在线阅读,更多相关《鼻腔和口腔吸入制剂指导原则-EMA(翻译版).pdf(42页珍藏版)》请在冰豆网上搜索。
7WestferryCircus,CanaryWharf,London,E144HB,UKTel.(44-20)74188400Fax(44-20)74188595E-mail:
mailemea.eu.inthttp:
/www.emea.eu.intEMEA2006Reproductionand/ordistributionofthisdocumentisauthorisedfornoncommercialpurposesonlyprovidedtheEMEAisacknowledgedEuropeanMedicinesAgencyInspectionsLondon,21June2006DocRef.:
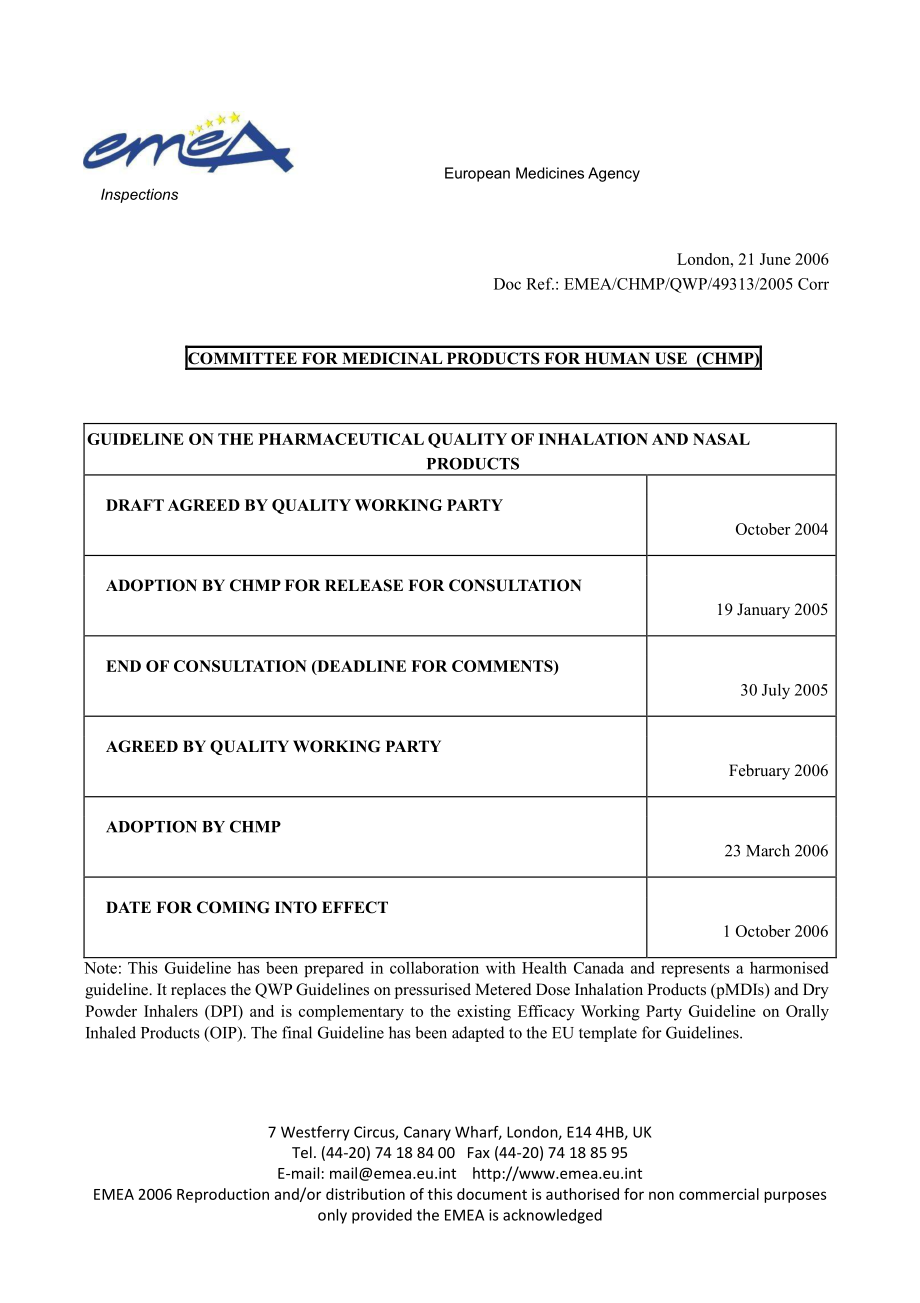
EMEA/CHMP/QWP/49313/2005CorrCOMMITTEEFORMEDICINALPRODUCTSFORHUMANUSE(CHMP)GUIDELINEONTHEPHARMACEUTICALQUALITYOFINHALATIONANDNASALPRODUCTSDRAFTAGREEDBYQUALITYWORKINGPARTYOctober2004ADOPTIONBYCHMPFORRELEASEFORCONSULTATION19January2005ENDOFCONSULTATION(DEADLINEFORCOMMENTS)30July2005AGREEDBYQUALITYWORKINGPARTYFebruary2006ADOPTIONBYCHMP23March2006DATEFORCOMINGINTOEFFECT1October2006Note:
ThisGuidelinehasbeenpreparedincollaborationwithHealthCanadaandrepresentsaharmonisedguideline.ItreplacestheQWPGuidelinesonpressurisedMeteredDoseInhalationProducts(pMDIs)andDryPowderInhalers(DPI)andiscomplementarytotheexistingEfficacyWorkingPartyGuidelineonOrallyInhaledProducts(OIP).ThefinalGuidelinehasbeenadaptedtotheEUtemplateforGuidelines.MEA/CHMP/QWP/49313/2005CorrEMEA2006GUIDELINEONTHEPHARMACEUTICALQUALITYOFINHALATIONANDNASALPRODUCTSTABLEOFCONTENTSCOMMITTEEFORMEDICINALPRODUCTSFORHUMANUSE(CHMP).11.INTRODUCTION.12.SCOPE.14.1DRUGSUBSTANCESPECIFICATION.24.2DRUGPRODUCTPHARMACEUTICALDEVELOPMENT.34.2.1InhalationProducts.44.2.1.1Physicalcharacterisation(CTD3.2.P.2.1.1and3.2.P.2.1.2).74.2.1.2MinimumFillJustification(CTD3.2.P.2.2.2).74.2.1.3Extractables/Leachables(CTD3.2.P.2.4).74.2.1.4Delivereddoseuniformityandfineparticlemassthroughcontainerlife(CTD3.2.P.2.4).84.2.1.5Delivereddoseuniformityandfineparticlemassoverpatientflowraterange(CTD3.2.P.2.4)94.2.1.6Fineparticlemasswithspacer/holdingchamberuse(CTD3.2.P.2.4).94.2.1.7Singledosefineparticlemass(CTD3.2.P.2.4).104.2.1.8Particle/dropletsizedistribution(CTD3.2.P.2.4).114.2.1.9Actuator/Mouthpiecedeposition(CTD3.2.P.2.4).114.2.1.10Drugdeliveryrateandtotaldrugdelivered(CTD3.2.P.2.4).124.2.1.11Shakingrequirements(CTD3.2.P.2.4).124.2.1.12Initialprimingofthecontainer(CTD3.2.P.2.4).124.2.1.13Re-primingofthecontainer(CTD3.2.P.2.4).124.2.1.14Cleaningrequirements(CTD3.2.P.2.4).134.2.1.15Lowtemperatureperformance(CTD3.2.P.2.4).134.2.1.16Performanceaftertemperaturecycling(CTD3.2.P.2.4).144.2.1.17Effectofenvironmentalmoisture(CTD3.2.P.2.4).144.2.1.18Robustness(CTD3.2.P.2.4).144.2.1.19Deliverydevicedevelopment(CTD3.2.P.2.4and3.2.R).154.2.1.20Preservativeeffectiveness/efficacy(CTD3.2.P.2.5).164.2.1.21Compatibility(CTD3.2.P.2.6).164.2.2NasalProducts.164.3DRUGPRODUCTMANUFACTURE.194.4EXCIPIENTS.194.4.1PharmacopoeialExcipients.204.4.2Non-PharmacopoeialExcipients.214.5DRUGPRODUCTSPECIFICATION(S).214.5.1InhalationProducts.224.5.1.1Description.234.5.1.2Assay.234.5.1.3MoistureContent.234.5.1.4MeanDeliveredDose.244.5.1.5DeliveredDoseUniformity.244.5.1.6ContentUniformity/UniformityofDosageUnits.24MEA/CHMP/QWP/49313/2005CorrEMEA20064.5.1.7FineParticleMass.244.5.1.8LeakRate.254.5.1.9Microbial/MicrobiologicalLimits.254.5.1.10Sterility.254.5.1.11Leachables.254.5.1.12Preservativecontent.264.5.1.13Numberofactuationspercontainer.264.5.2NasalProducts.264.5.2.1Particle/DropletSizeDistribution.264.6DRUGPRODUCTCONTAINERCLOSURESYSTEM.284.7DRUGPRODUCTSTABILITY.29APPENDIXI:
.33APPENDIXII:
.36APPENDIXIII:
.39MEA/CHMP/QWP/49313/2005CorrEMEA2006page1/39译文:
魏利军邮箱:
1.INTRODUCTION介绍介绍Thisguidancedocumentappliestohumanmedicinalproductsintendedfordeliveryofthedrugsubstanceintothelungs,ortothenasalmucosa,withthepurposeofevokingalocalorsystemiceffect.该指南文件适用于递送药物到肺部或鼻腔引起局部或全身效应的人用药品。
Thedocumentoutlinesexpectedqualityaspectsofdrugproductstobemarketed,butthegeneralprinciplesdescribedhereshouldalsobeconsideredforproductsusedinclinicaltrials.Itisnotexpectedthatalldescribedtestingwouldbeconductedonallclinicaltrialbatches.However,extensivecharacterisationofthedrugsubstanceanddrugproductbatchesusedinpivotalclinicaltrialsisnecessarytoqualifytheproductproposedformarketing.文件概述了上市药品预期的质量,对于临床试验所用药品,此文件描述的一般原则也应加以考虑。
并不希望每一批用于临床试验的药品都开展每一项所述的测试,然而在关键性临床试验中,广泛地表征药品及其原料药以明确其上市后的特性是有必要的。
Onlyqualityaspectsspecifictoinhalationandnasalproductsarediscussed,althoughtheneedforsafetytesting(e.g.,forexcipientsandleachables)isalsoaddressed.Additionalqualityaspects(e.g.,impurities,processvalidation,stabilitytesting,specifications)aswellassafetyandefficacyaspects,aredescribedinotherguidancedocuments,includingICHguidelines.在此只对吸入制剂和鼻药产品质量方面的特殊问题进行讨论,尽管安全性测试(如辅料和析出物)也亟待解决。
更多质量方面(如杂质、工艺验证、稳定性试验、质量标准)、安全性方面和有效性方面的内容,已在其它指南中描述,包括ICH指南。
Detailedguidanceonpharmaceuticaldevelopmentstudydesigns(e.g.,primingstudies)andtheanalyticalproceduresusedprimarilyforinhalationandnasalproducts(e.g.,cascadeimpactoranalysis)hasnotbeenprovided.Someofthisinformationmaybefoundinotherpublications(e.g.,UnitedStatesPharmacopeia,EuropeanPharmacopoeia,ISOstandards).Itisalsorecognisedthatthewidediversityofinhalationandnasalproductswithrespecttoformulationanddeliverydevicecharacteristicsnecessitatessomeflexibilityintestingmethodology.有关药学开发研究设计(如灌注研究)和主要用于吸入制剂和鼻药产品的分析过程的详细指南已在文中列出,其中某些部分信息也可能在其它出版物(如美国药典、欧洲药典、国际标准)中发现。
人们已意识到吸入制剂和鼻药产品在处方和递送装置特性的巨大差异,而这种差异早就了测试方法学的灵活性。
2.SCOPE范围范围Thedocumentaddressesnewmarketingauthorisationapplications(includingforgenericproducts)anddoesnotoutlineexpectedqualityaspectsrelatedtochangesinexistinginhalationandnasalproducts.However,thegeneralprinciplesdescribedhereshouldalsobeconsideredwhenmakingchangestoexistingproducts.MEA/CHMP/QWP/49313/2005CorrEMEA2006page2/39译文:
魏利军邮箱:
文件提出新药上市批准申请(也包括仿制药品),并没有作有关吸入制剂和鼻药产品的质量方面发生变化的概述。
然而在当更改现有的产品时,此处描述的一般原则也应考虑。
Thisguidancedocumenthasbeendevelopedforproductscontainingdrugsubstancesofsyntheticorsemi-syntheticorigin.However,thegeneralprinciplesdescribedhereshouldalsobeconsideredforotherinhalationandnasalproducts.对于含合成和半合成原料药来源的产品,该指南文件已经发展成熟。
然而对于其它类型的吸入制剂和鼻药产品,此处描述的一般原则应该考虑。
Thisdocumentincludesproductsforadministrationofthedrugsubstancetothelungs,suchaspressurisedmetereddoseinhalers,drypowderinhalers,productsfornebulisation,andnonpressurisedmetereddoseinhalers,aswellaspressurisedmetereddosenasalsprays,nasalpowders,andnasalliquids.Liquidinhalationanaestheticsandnasalointments,creamsandgelsareexcluded.该文件涵盖了除液体吸入型麻醉剂、鼻腔软膏、霜剂和凝胶剂以外的各种肺部给药产品,如加压计量型气雾剂、干粉吸入剂、喷雾剂、非加压计量型气雾剂、加压计量型鼻腔气雾剂、鼻腔粉剂、滴鼻液。
3.LEGALBASISDirective2001/83/EC,asamended.4.MAINGUIDELINETEXT4.1DRUGSUBSTANCESPECIFICATION原料药标准Forallinhalationandnasalproductscontainingadrugsubstancethatisnotinsolutionatanytimeduringdrugproductmanufacture,storageoruse,thedrugsubstancespecificationshouldincludeaparticlesizetestandlimits.Avalidatedparticlesizingmethod(e.g.,laserdiffraction),withacceptancecriteriasetatmultiplepointsacrossthesizedistribution,shouldbeemployed.所有吸入剂和鼻药产品在生产、储存或使用过程中原料药并非一直是溶液状态存在,原料药质量标准中应规定粒度检测项和相应的限度,应使用经过验证的粒度测定方法(比如激光散射)进行粒度检测,而且在粒度分布范围内应指定多个粒径下的检验标准。
Acceptancecriteriashouldassureaconsistentparticlesizedistributionintermsofthepercentageoftotalparticlesingivensizeranges.Themedian,upper,and/orlowerparticlesizelimitsshouldbewell-defined.Acceptancecriteriashouldbesetbasedontheobservedrangeofvariation,andshouldtakeintoaccounttheparticlesizedistributionofbatchesthatshowedacceptableperformanceinvivo,aswellastheintendeduseoftheproduct.Processcapabilityandstabilitydatamayalsobeconsidered,providedtheproposedacceptancecriteriahavebeensuitablyqualified.MEA/CHMP/QWP/49313/2005CorrEMEA2006page3/39译文:
魏利军邮箱:
检验标准应包括在规定粒度范围下粒子数占总粒子数的百分比,中间,最大和最小等粒度限度都需要明确规定。
检验标准的确定应基于实测的粒度范围差异之上,综合考虑多批不同粒度分布的产品在体内表现出的可接受性能差异,其也包括将要用在临床上的产品。
工艺的批量和稳定性数据也应该关注,提供的拟定标准应经过适当资质的确证。
Ifalternativesourcesofdrugsubstanceareproposed,evidenceofequivalenceshouldincludeappropriatephysicalcharacterisationandinvitroperformancestudies(seealsoDrugProductPharmaceuticalDevelopment).如果是申请变更原料药来源,应提供包括适当的物理性质和体外性能研究(参见药品药物开发)在内的等效性证据。
4.2DRUGPRODUCTPHARMACEUTICALDEVELOPMENT药品开发Pharmaceuticaldevelopmentstudiesareconductedtoestablishthatthedosageform,formulation,manufacturingprocess,containerclosuresystem,microbiologicalattributesandinstructionsforuseareappropriateandresultinacceptableproductperformance.药品开发是指确立剂型,处方,工艺,储药器和密封系统,微生物属性,合理使用说明的整个过程,以及符合以上要求的产品性能。
Itisgenerallyexpectedthatthedevelopmenttestsbeconductedonmorethanonebatch,sothatbatchvariabilityistakenintoaccount.Forasinglestrengthandasinglecontainerclosuresystem,testingtwobatchesshouldbesufficient.Forproductspackagedincontainerclosuresystemsthatalsoserveasthedeliverydevice,teststhatinvolvedeliveryoftheformulationshouldalsobeconductedonmorethanonebatchofthecontainerclosuresystem.Inthecaseofmultiplestrengthsandmultiplepackagesizes,abracketingand/ormatrixingdesignmaybeusedtolimitthenumberoftestsamplesnecessary.Justificationshouldbeprovided.普遍认为药品开发时应对多批产品进行检验,以分析批间的差异。
对于单规格和单储药器的产品,检测两批即可。
对于产品密封在递送装置中的多剂量型产品,应在多批次储药器的基础上研究递送配方的性能。
如果是多个规格和多个包装尺寸的产品,有必要用括号法或矩阵化设计来限制样本数量,同时提供相应的理由。
Sufficientdatashouldbeprovidedtosupportthespecificationsproposedortogiveadequateassurancethatthoseperformancecharacteristicswhichmaynotberoutinelytested(e.g.,primingandtestingtoexhaustion)havebeenadequatelyinvestigated.It