标准规定电极电势表Word格式.docx
《标准规定电极电势表Word格式.docx》由会员分享,可在线阅读,更多相关《标准规定电极电势表Word格式.docx(14页珍藏版)》请在冰豆网上搜索。
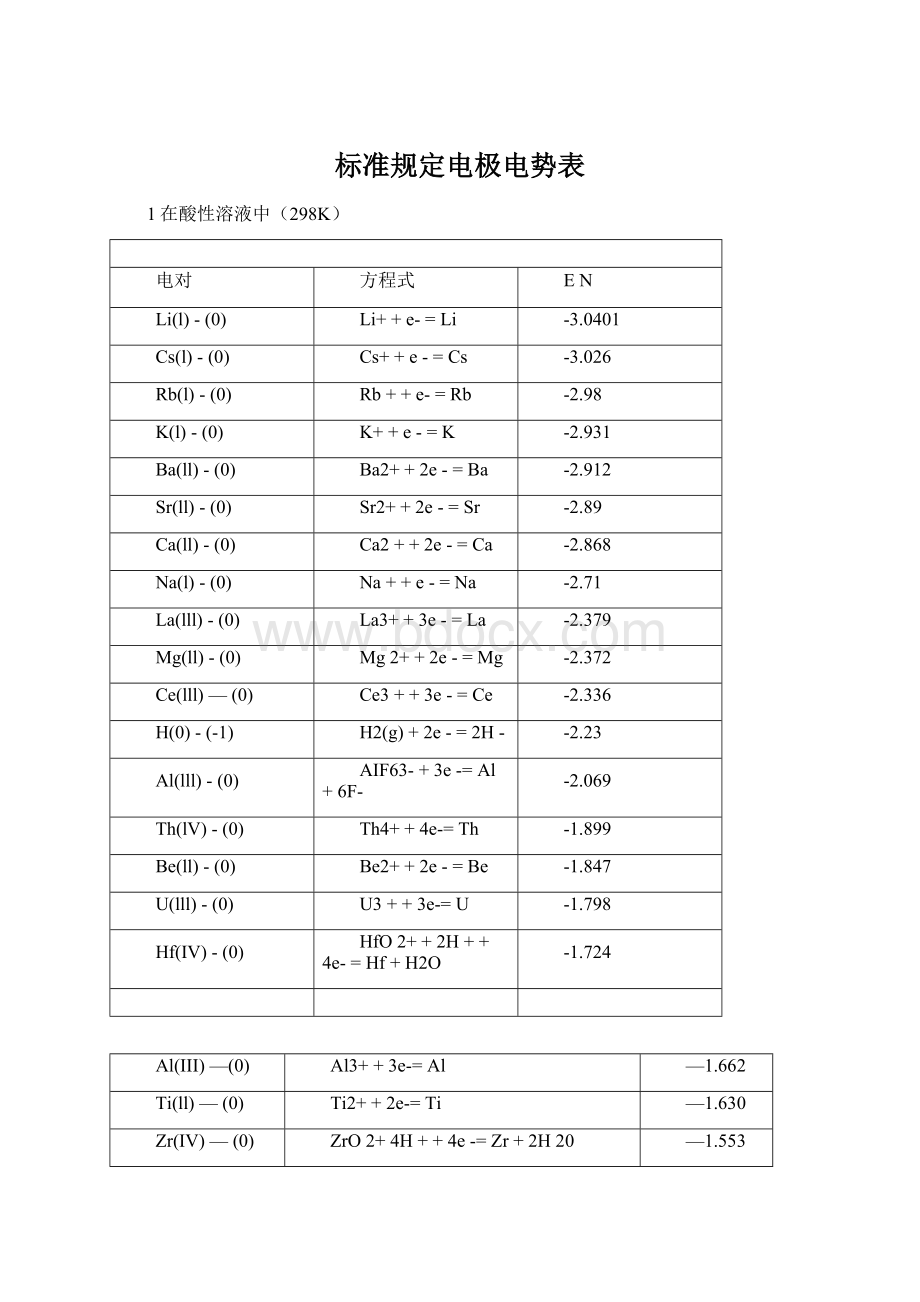
Al3++3e-=Al
—1.662
Ti(ll)—(0)
Ti2++2e-=Ti
—1.630
Zr(IV)—(0)
ZrO2+4H++4e-=Zr+2H20
—1.553
Si(IV)—(0)
[SiF6]2-+4e-=Si+6F—
—1.24
Mn(ll)—(0)
Mn2++2e-=Mn
—1.185
Cr(ll)—(0)
Cr2++2e-=Cr
—0.913
Ti(lll)—(ll)
Ti3++e-=Ti2+
—0.9
B(lll)—(0)
H3BO3+3H++3e-=B+3H2O
—0.8698
*Ti(lV)—(0)
TiO2+4H++4e-=Ti+2H2O
—0.86
Te(0)—(—ll)
Te+2H++2e-=H2Te
—0.793
Zn(ll)—(0)
Zn2++2e-=Zn
—0.7618
Ta(V)—(0)
Ta2O5+10H++10e—=2Ta+5H2O
—0.750
Cr(lll)—(0)
Cr3++3e-=Cr
—0.744
Nb(V)—(0)
Nb2O5+l0H++10e-=2Nb+5H2O
—0.644
As(0)—(—lll)
As+3H++3e-=AsH3
—0.608
U(lV)—(lll)
U4++e-=U3+
—0.607
Ga(lll)—(0)
Ga3++3e-=Ga
—0.549
P(l)—(0)
H3PO2+H++e-=P+2H2O
—0.508
P(lll)—(l)
H3PO3+2H++2e-=H3PO2+H2O
—0.499
*C(IV)—(III)
2CO2+2H++2e-=H2C2O4
—0.49
Fe(ll)—(0)
Fe2++2e-=Fe
—0.447
Cr(lll)—(II)
Cr3++e-=Cr2+
—0.407
Cd(ll)—(0)
Cd2++2e-=Cd
—0.4030
Se(0)—(—ll)
Se+2H++2e-=H2Se(aq)
—0.399
Pb(ll)—(0)
Pbl2+2e-=Pb+2l—
—0.365
Eu(lll)—(ll)
Eu3++e-=Eu2+
—0.36
PbSO4+2e-=Pb+SO42—
—0.3588
ln(lll)—(0)
ln3++3e-=ln
—0.3382
Tl(l)—(0)
Tl++e-=Tl
—0.336
Co(ll)—(0)
Co2++2e-=Co
—0.28
P(V)—(lll)
H3PO4+2H++2e-=H3PO3+H2O
—0.276
PbCl2+2e-=Pb+2Cl—
—0.2675
Ni(ll)—(0)
Ni2++2e-=Ni
—0.257
V(lll)—(ll)
V3++e-=V2+
—0.255
Ge(lV)—(0)
H2GeO3+4H++4e-=Ge+3H2O
—0.182
Ag(l)—(0)
Agl+e—=Ag+l—
—0.15224
Sn(ll)—(0)
Sn2++2e-=Sn
—0.1375
Pb2++2e-=Pb
—0.1262
*C(IV)-(II)
CO2(g)+2H++2e-=CO+出0
—0.12
P(0)-(—III)
P(white)+3H++3e—=PH3(g)
—0.063
Hg(I)-(0)
Hg2b+2e—=2Hg+2I—
—0.0405
Fe(III)-(0)
Fe3++3e-=Fe
—0.037
H(I)-(0)
2H++2e-=H2
0.0000
Ag(I)-(0)
AgBr+e—=Ag+Br—
0.07133
S(II.V)-(II)
S4O62—+2e-=2S2O32—
0.08
*Ti(IV)—(III)
TiO2++2H++e-=Ti3++H2O
0.1
S(0)—(—II)
S+2H++2e-=H2S(aq)
0.142
Sn(IV)—(II)
Sn4++2e-=Sn2+
0.151
Sb(III)—(0)
Sb2O3+6H++6e-=2Sb+3H2O
0.152
Cu(II)—(I)
Cu2++e-=Cu+
0.153
Bi(III)—(0)
BiOCl+2H++3e-=Bi+Cl—+H2O
0.1583
S(VI)—(IV)
SO42—+4H++2e-=H2SO3+H2O
0.172
SbO++2H++3e-=Sb+H2O
0.212
Ag(I)—(0)
AgCl+e——Ag+Cl—
0.22233
As(III)—(0)
HAsO2+3H++3e——As+2H2O
0.248
Hg(I)—(0)
Hg2Cl2+2e——2Hg+2Cl—(饱和KCl)
0.26808
BiO++2H++3e——Bi+H2O
0.320
U(VI)—(IV)
UO22++4H++2e-=U4++2H2O
0.327
C(IV)—(III)
2HCNO+2H++2e-=(CN)2+2H20
0.330
V(IV)—(III)
VO2++2H++e-=V3++H2O
0.337
Cu(II)—(0)
Cu2++2e-=Cu
0.3419
Re(VII)—(0)
ReO4—+8H++7e-=Re+4H2O
0.368
Ag2CrO4+2e-=2Ag+CrO42—
0.4470
S(IV)—(0)
H2SO3+4H++4e-=S+3H2O
0.449
Cu(I)—(0)
Cu++e-=Cu
0.521
I(0)—(—I)
I2+2e—=2I—
0.5355
l3-+2e—=3I—
0.536
As(V)—(III)
H3ASO4+2H++2e-=HASO2+2H2O
0.560
Sb(V)—(III)
Sb2O5+6H++4e-=2SbO++3H2O
0.581
Te(IV)—(0)
TeO2+4H++4e-=Te+2H2O
0.593
U(V)—(IV)
UO2++4H++e-=U4++2H2O
0.612
**Hg(II)—(I)
2HgCl2+2e—=Hg2CI2+2Cl—
0.63
Pt(IV)—(II)
[PtCI6]2—+2e-=[PtCl4]2—+2Cl—
0.68
0(0)—(—I)
O2+2H++2e-=H2O2
0.695
Pt(II)—(0)
[PtCl4]2—+2e—=Pt+4Cl—
0.755
*Se(IV)—(0)
H2SeO3+4H++4e-=Se+3H2O
0.74
Fe(III)—(II)
Fe3++e-=Fe2+
0.771
Hg(l)—(0)
Hg22++2e-=2Hg
0.7973
Ag++e_=Ag
0.7996
Os(VIII)—(0)
OsO4+8H++8e-=Os+4H2O
0.8
N(V)—(IV)
2NO3—+4H++2e-=N2O4+2H2O
0.803
Hg(II)—(0)
Hg2++2e_=Hg
0.851
(quartz)SiO2+4H++4e-=Si+2H2O
0.857
Cu2++I—+e—=CuI
0.86
N(III)—(I)
2HNO2+4H++4e-=H2N2O2+2H2O
Hg(II)—(I)
2Hg2++2e-=Hg22+
0.920
N(V)—(III)
NO3—+3H++2e-=HNO2+H2O
0.934
Pd(II)—(0)
Pd2++2e-=Pd
0.951
N(V)—(II)
NO3—+4H++3e-=NO+2H2O
0.957
N(III)—(II)
HNO2+H++e-=NO+H2O
0.983
I(I)—(—I)
HIO+H++2e-=I—+H2O
0.987
V(V)—(IV)
VO2++2H++e-=VO2++H2O
0.991
V(OH)4++2H++e-=VO2++3H2O
1.00
Au(III)—(0)
[AuCl4]—+3e—=Au+4Cl—
1.002
Te(VI)—(IV)
H6TeO6+2H++2e—=TeO2+4H2O
1.02
N(IV)—(II)
N2O4+4H++4e-=2NO+2H2O
1.035
N(IV)—(III)
N2O4+2H++2e-=2HNO2
1.065
I(V)—(—I)
IO3—+6H++6e—=I—+3H2O
1.085
Br(0)—(—I)
Br2(aq)+2e—=2Br—
1.0873
Se(VI)—(IV)
SeO42—+4H++2e—=H2SeO3+H2O
1.151
CI(V)—(IV)
CIO3—+2H++e-=CIO2+H2O
1.152
Pt2++2e-=Pt
1.18
CI(VII)—(V)
CIO4—+2H++2e-=CIO3—+H2O
1.189
I(V)—(0)
2IO3—+12H++10e—=I2+6H2O
1.195
CI(V)—(III)
CIO3—+3H++2e-=HCIO2+H2O
1.214
Mn(IV)—(II)
MnO2+4H++2e-=Mn2++2H2O
1.224
O(0)—(—II)
O2+4H++4e-=2H2O
1.229
TI(III)—(I)
T13++2e—=Tl+
1.252
CI(IV)—(III)
CIO2+H++e-=HCIO2
1.277
2HNO2+4H++4e-=N2O+3H2O
1.297
**Cr(VI)—(III)
Cr2O72—+14H++6e—=2Cr3++7H2O
1.33
Br(I)—(—I)
HBrO+H++2e-=Br—+H2O
1.331
Cr(VI)—(III)
HCrO4—+7H++3e—=Cr3++4H2O
1.350
CI(0)—(—I)
Cb(g)+2e-=2CI—
1.35827
CI(VII)—(-I)
CI04—+8H++8e—=Cl—+4H2O
1.389
CI(VII)—(0)
CI04—+8H++7e—=1/2CI2+4H2O
1.39
Au(III)—(I)
Au3++2e—=Au+
1.401
Br(V)—(—I)
BrO3—+6H++6e-=Br—+3H2O
1.423
I(I)—(0)
2HIO+2H++2e-=I2+2H2O
1.439
CI(V)—(—I)
CIO3—+6H++6e—=CI—+3H2O
1.451
Pb(IV)—(II)
PbO2+4H++2e-=Pb2++2H2O
1.455
CI(V)—(0)
CIO3—+6H++5e—=1/2CI2+3H2O
1.47
CI(I)—(—I)
HCIO+H++2e-=CI—+H2O
1.482
Br(V)—(0)
BrO3—+6H++5e—=I/2Br2+3H2O
Au3++3e-=Au
1.498
Mn(VII)—(II)
MnO4—+8H++5e-=Mn2++4H2O
1.507
Mn(HI)—(II)
Mn3++e-=Mn2+
1.5415
CI(III)—(—I)
HCIO2+3H++4e-=Cl—+2H2O
1.570
Br(I)—(0)
HBrO+H++e—=I/2Br2(aq)+H2O
1.574
N(II)—(I)
2NO+2H++2e-=N2O+H2O
1.591
I(VII)—(V)
H5IO6+H++2e—=IO3—+3H2O
1.601
CI(I)—(0)
HCIO+H++e-=1/2CI2+H2O
1.611
CI(III)—(I)
HCIO2+2H++2e-=HCIO+H2O
1.645
Ni(IV)—(II)
NiO2+4H++2e-=Ni2++2H2O
1.678
Mn(VII)—(IV)
MnO4—+4H++3e-=MnO2+2H2O
1.679
PbO2+SO42—+4H++2e-=PbSO4+2H2O
1.6913
Au(I)—(0)
Au++e—=Au
1.692
Ce(IV)—(III)
Ce4++e—=Ce3+
1.72
N(I)—(0)
N2O+2H++2e-=N2+H2O
1.766
O(—I)—(—II)
H2O2+2H++2e-=2H2O
1.776
Co(III)—(II)
Co3++e-=Co2+(2molL—1H2SO4)
1.83
Ag(II)—(I)
Ag2++e_=Ag+
1.980
S(VII)—(VI)
S2O82—+2e—=2SO42—
2.010
O3+2H++2e-=O2+H2O
2.076
O(II)—(—II)
F2O+2H++4e-=H2O+2F—
2.153
Fe(VI)—(III)
FeO42—+8H++3e—=Fe3++4H2O
2.20
O(g)+2H++2e-=H2O
2.421
F(0)—(—I)
F2+2e—=2F—
2.866
F2+2H++2e-=2HF
3.053
2在碱性溶液中(298K)
E/V
Ca(OH)2+2e-=Ca+2OH-
-3.02
Ba(OH)2+2e-=Ba+2OH-
-2.99
La(OH)3+3e-=La+3OH-
-2.90
Sr(OH)28H2O+2e-=Sr+2OH-+8H2O
-2.88
Mg(OH)2+2e-=Mg+2OH-
-2.690
Be2O32-+3H2O+4e-=2Be+6OH-
-2.63
Hf(IV)—(0)
HfO(OH)2+H2O+4e-=Hf+4OH-
-2.50
Zr(lV)-(0)
H2ZrO3+H2O+4e-=Zr+4OH-
-2.36
H2AlO3-+H2O+3e-=Al+OH-
-2.33
P(l)-(0)
H2PO2-+e-=P+2OH-
-1.82
B(lll)-(0)
H2BO3-+H2O+3e-=B+4OH-
-1.79
P(lll)-(0)
HPO32-+2H2O+3e-=P+5OH-
-1.71
Si(lV)-(0)
SiO32-+3H2O+4e-=Si+6OH-
-1.697
P(lll)-(l)
HPO32-+2H2O+2e-=H2PO2-+3OH-
-1.65
Mn(ll)-(0)
Mn(OH)2+2e-=Mn+2OH-
-1.56
Cr(lll)-(0)
Cr(OH)3+3e-=Cr+3OH-
-1.48
*Zn(ll)-(0)
[Zn(CN)4]2-+2e-=Zn+4CN-
-1.26
Zn(ll)-(0)
Zn(OH)2+2e-=Zn+2OH-
-1.249
Ga(lll)-(0)
H2GaO3-+H2O+2e-=Ga+4OH-
-1.219
Zn(II)—(0)
ZnO22-+2H2O+2e-=Zn+4OH—
—1.215
CrO2—+2H2O+3e—=Cr+4OH—
—1.2
Te(0)—(—I)
Te+2e—=Te2-
—1.143
P(V)—(Ill)
PO43—+2H2O+2e-=HPO32—+3OH—
—1.05
*Zn(II)—(0)
[Zn(NH3)4]2++2e-=Zn+4NH3
—1.04
*W(VI)—(0)
WO42—+4H2O+6e-=W+8OH—
—1.01
*Ge(IV)—(0)
HGeO3—+2H2O+4e—=Ge+5OH—
—1.0
[Sn(OH)6]2—+2e-=HSnO2-+H2O+3OH—
—0.93
SO42—+H2O+2e—=SO32—+2OH—
Se(0)—(—II)
Se+2e-=Se2—
—0.924
Sn(II)—(0)
HSnO2-+H2O+2e-=Sn+3OH-
—0.909
P(0)—(—Ill)
P+3H2O+3e-=PH3©
+3OH—
—0.87
2NO3—+2H2O+2e-=N2O4+4OH—
—0.85
H(l)—(0)
2H2O+2e-=H2+2OH—
—0.8277
C