中山大学生物化学课件.ppt
《中山大学生物化学课件.ppt》由会员分享,可在线阅读,更多相关《中山大学生物化学课件.ppt(85页珍藏版)》请在冰豆网上搜索。
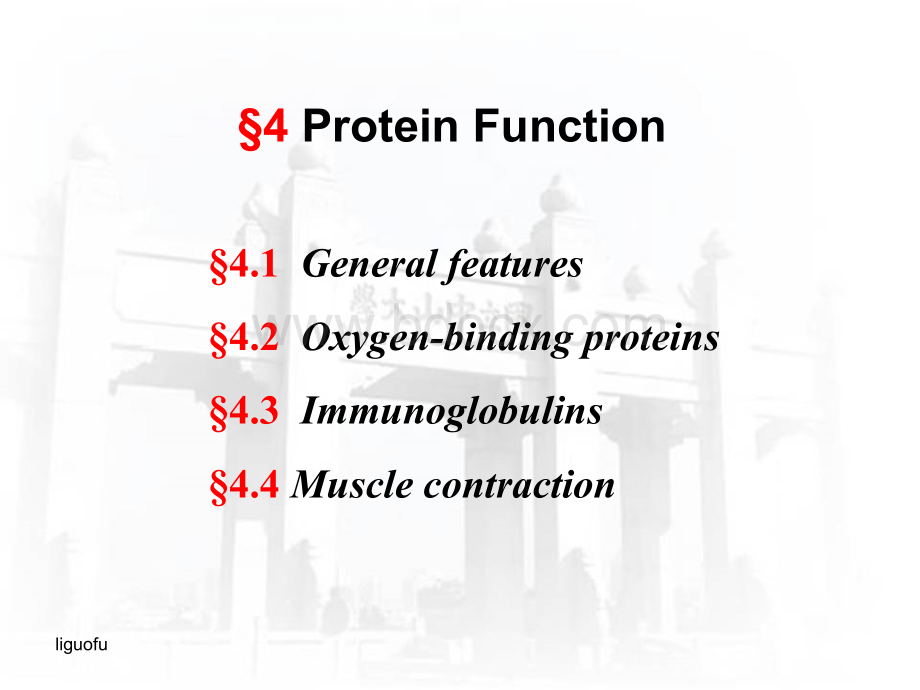
4ProteinFunction4.1Generalfeatures4.2Oxygen-bindingproteins4.3Immunoglobulins4.4Musclecontractionliguofu4.1Generalfeatures1.Versatileinfunction2.Beinghardtostudy3.FunctionviainteractionTheinteractionwithothermolecule(ligand)isreversible.Theinterfacebetweenthebindingsiteiscomplementaryinstructure,makingsuchinteractionhighlyspecific.Structuredynamicnessofaproteinisusuallyessentialforsuchinteractions.liguofu4.1Generalfeatures4.2Oxygen-bindingproteins4.3Immunoglobulins4.4Musclecontraction4ProteinFunctionliguofuQuantitativedescriptionofinteraction
(1)Protein+nLigandPLnliguofuQuantitativedescriptionofinteraction
(2)IfkdisconstantliguofuQuantitativedescriptionofinteraction(3)IfkdisnotconstantKdliguofuQuantitativedescriptionofinteraction(4)liguofuQuantitativedescriptionofinteraction(5)liguofuTheiron-porphyrininhemoglobinaccountsfortheredcolorofblood,thecopper-porphyrininhemocyaninforbluecolorofblood,andthemagnesium-porphyrininchlorophyllisresponsibleforthegreenofplants.liguofuStructureofPorphyrinPyrroleringMethenebridgeliguofuOxygencanbeboundtoaheme
(1)Heme=ProtoporphyrinIX+Fe2+NoneoftheaasidechainsinproteinsissuitedforreversiblebindingO2HemeprostheticgroupTransitionmetals,Fe&Cu,haveastrongtendencytobindO2needmorecommonlyliguofuOxygencanbeboundtoaheme
(2)Heme=ProtoporphyrinIX+Fe2+lFreeIronpromotestheformationofhighlyreactiveoxygenspeciessuchashydroxylradicals.lCoordinatedNatomshelppreventtheconversionofFe2+toFe3+liguofuMbhasasinglebindingsiteforoxygenMyoglobin:
153residues,16700DaHis93/F8His64/E7liguofuOxygencanbeboundtoaheme(3)lInfreehememolecules,reactionofoxygenatoneofthetwo“open”coordinationbondsofironcanresultinirreversibleconversionofFe2+toFe3+lInheme-containingproteins,thisreactionispreventedbysequesteringthehemedeepwithinaproteinstructurewhereacesstothetwo“open”coordinationbondsisrestricted.Oneofthesetwocoordinationbondsisoccupiedbyaside-chainNofaHisresidue.NOCOAlsoliguofuQuantitativedescriptionofMbbindingO2liguofuProteinstructureaffectsligandsbind
(1)InfreehemeorInmyoglobinInfreehemeInmyoglobinhemeorliguofu“Breathing”:
MolecularmotionsThebindingofO2tohemeinmyoglobindependsonits“breathing”InmyoglobinProteinstructureaffectsligandsbind
(2)liguofuHbisthemost-studiedandbest-understoodprotein1.Hemoglobinwasthefirstproteintobecrystallized(in1849);thefirsttobeassociatedwithaspecificphysiologicalfunction(around1875);oneofthefirstproteinstohaveitsmolecularweightdeterminedcorrectly(64,500);thefirsteukaryoticmessenger(mRNA)tobeisolatedandsubsequentlysequenced.thefirsteukaryoticproteintobesynthesizedinacell-freesysteminvitro;thesecondproteinhavingits3-Dstructuredetermined(1969).liguofu2.The“redcoloringmatter”(hemoglobin)ofanimalbloodcouldbebroughttocrystallization,withcrystalformscharacteristicoftheirbiologicalorigins(1840s-1860s).3.Reversibleinterconversionofoxyhemoglobinanddeoxyhemoglobinwasrevealedbyusingspectroscope(1860s).4.Treatmentofhemoglobinwithacidgaveacolorlessalbuminoidconstituent(globin)andarediron-containingmaterial(by1870).5.Thestructureofhemewaselucidatedtobeatetrapyrrol(porphyrin)derivativebychemicalsynthesis(HansFischer,1929).6.Similartetrapyrrolderivativesarealsousedasprostheticgroupsofotherproteins(e.g.,thecytochromesthatfunctioninbiologicaloxidationandphotosynthesis!
liguofuHbhasfoursubunitsMr=64,500141residues146residuesliguofuHbsubunitsarestructurallysimilartomyoglobinsubunitlackstheshortDhelixDhelixliguofuconservedinallknownglobinsconserved27positionsareidentical,18%82143liguofu30residuesInteractionsinthefoursubunits19residues19residuesHydrophobicinteractionsHbondsSaltbondsliguofuThisiscalledTensestate(deoxyHb)SomeionpairsarenotshownhereIonpairsatthe1/2and2/1interface
(1)liguofuIonpairsatthe1/2and2/1interface
(2)IonpairsstabilizetheTstateofdeoxyHbliguofuQuantitativedescriptionofHbbindingO2
(1)liguofuHillplotnH:
hillcoefficientQuantitativedescriptionofHbbindingO2(3)liguofuHbundergoesastructuralchangeonbindingO2
(1)TensestateismorestableandthusthepredominantconformationofdeoxyHbRelaxedstateisthepredominantconformationofhigheraffinitytoO2BindingO2ValE11liguofuIntheTstate,theporphyrinisslightlypuckered,causingthehemeirontoprotrudesomewhatontheproximalHis(HisF8)side.ThebindingofO2causesthehemetoassumeamoreplanarconformation,shiftingthepositionofHisF8andFhelix.HbundergoesastructuralchangeonbindingO2
(2)liguofuAllostericproteinisoneinwhichthebindingofaligandtoonesiteaffectsthebindingpropertiesofanothersiteonthesameprotein.Modulatorisa