Organic chemistry 67.docx
《Organic chemistry 67.docx》由会员分享,可在线阅读,更多相关《Organic chemistry 67.docx(26页珍藏版)》请在冰豆网上搜索。
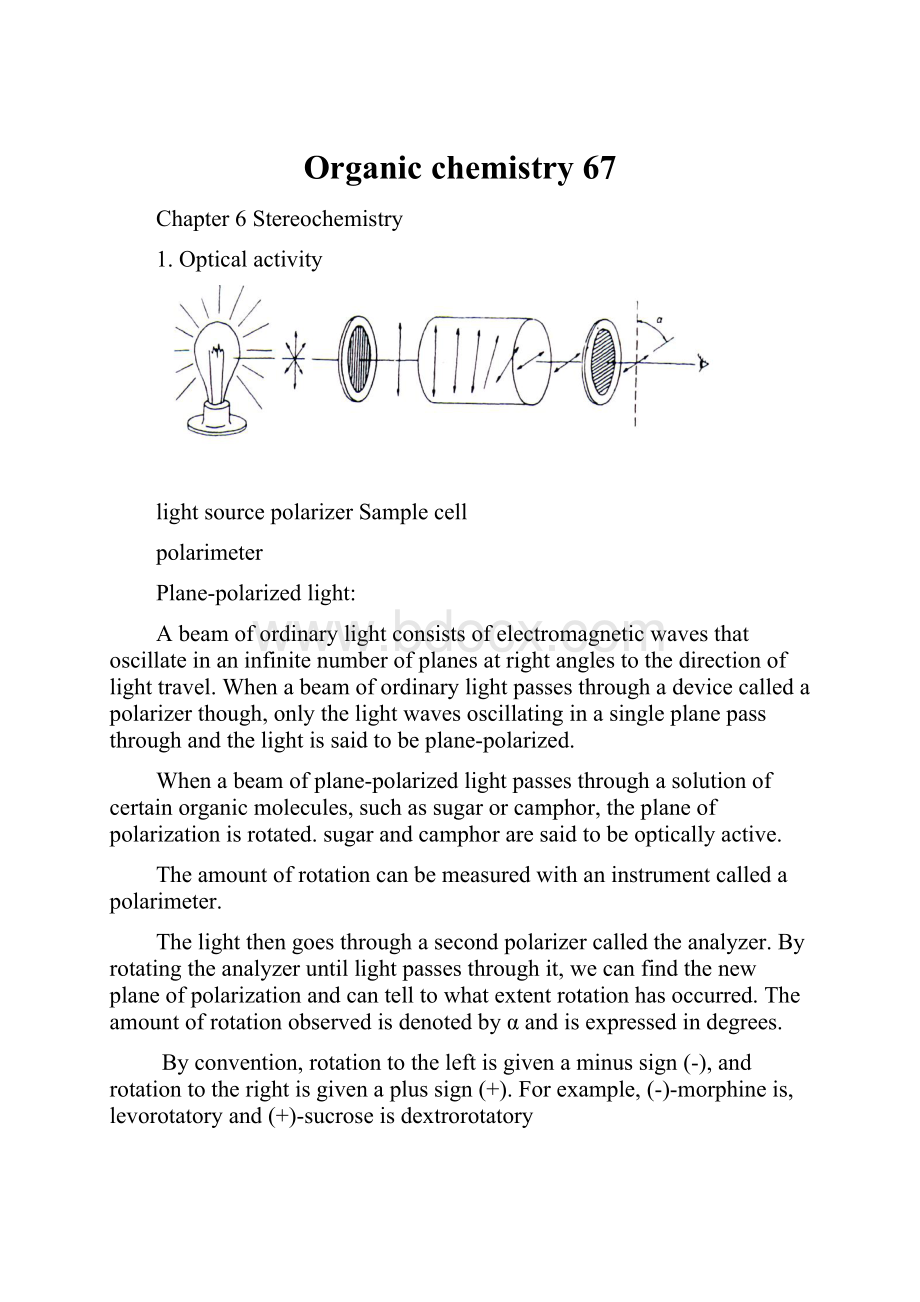
Organicchemistry67
Chapter6Stereochemistry
1.Opticalactivity
lightsourcepolarizerSamplecell
polarimeter
Plane-polarizedlight:
Abeamofordinarylightconsistsofelectromagneticwavesthatoscillateinaninfinitenumberofplanesatrightanglestothedirectionoflighttravel.Whenabeamofordinarylightpassesthroughadevicecalledapolarizerthough,onlythelightwavesoscillatinginasingleplanepassthroughandthelightissaidtobeplane-polarized.
Whenabeamofplane-polarizedlightpassesthroughasolutionofcertainorganicmolecules,suchassugarorcamphor,theplaneofpolarizationisrotated.sugarandcamphoraresaidtobeopticallyactive.
Theamountofrotationcanbemeasuredwithaninstrumentcalledapolarimeter.
Thelightthengoesthroughasecondpolarizercalledtheanalyzer.Byrotatingtheanalyzeruntillightpassesthroughit,wecanfindthenewplaneofpolarizationandcantelltowhatextentrotationhasoccurred.Theamountofrotationobservedisdenotedbyαandisexpressedindegrees.
Byconvention,rotationtotheleftisgivenaminussign(-),androtationtotherightisgivenaplussign(+).Forexample,(-)-morphineis,levorotatoryand(+)-sucroseisdextrorotatory
Inadditiontodeterminingtheextentofrotation,wecanalsofindthedirection.Fromthevantagepointoftheobserverlookingattheanalyzer,someopticallyactivemoleculesrotateplane-polarizedlighttotheleft(counterclockwise)andaresaidtobelevorotatory;othermoleculesrotatelighttotheright(clockwise)andaresaidtobedextrorotatory
opticalrotation(α)specificrotation[α]racemate(±),dl
t:
temperature(℃)
D:
sodiumlightsource(589nm)
l:
lengthofopticaltube(dm)
C:
concentration(g·ml-1)
2.Enantiomers
①Amoleculethatisnotidenticaltoitsmirrorimageisakindofstereoisomercalledanenantiomer;Enantiomersarerelatedtoeachotherasarighthandisrelatedtoalefthandandresultwheneveratetrahedralcarbonatomisbondedtofourdifferentsubstituents(oneneednotbeH).
②Chiral
Moleculesthatarenotidenticaltotheirmirrorimagesandthusexistintwoenantiomericformsaresaidtobechiral(Greekcheirmeaning"hand").Youcan'ttakeachiralmoleculeanditsmirrorimage(enantiomer)andplaceoneontopoftheothersothatallatomscoincide.
Themostcommon(althoughnottheonly)causeofchiralityinanorganicmoleculeisthepresenceofacarbonatombondedtofourdifferentgroups.
Suchcarbonsarereferredtoaschiralitycentersorstereocenters.
Notethatchiralityisapropertyoftheentiremolecule,whereasastereocenteristhecauseofchirality.
③Achiral(notchiral)
Amoleculeisnotchiralifitcontainsaplaneofsymmetry.Aplaneofsymmetryisaplanethatcutsthroughthemiddleofamoleculeorotherobjectsothatonehalfoftheobjectisanexactmirrorimageoftheotherhalf.
3.SequenceRulesforSpecifyingConfiguration.
Specifyingthethree-dimensionalarrangement,orconfiguration,ofsubstituentsaroundastereocenterisnecessary.
RULE1.Lookatthefouratomsdirectlyattachedtothestereocenterandassignprioritiesinorderofdecreasingatomicnumber.Theatomwiththehighestatomicnumberisrankedfirst;theatomwiththelowestatomicnumberisrankedfourth.
RULE2.Ifadecisioncan'tbereachedbyrankingthefirstatomsinthesubstituents,lookatthesecond,third,orfourthatomsoutwarduntilthefirstdifferenceisfound.
RULE3.Multiple-bondedatomsareequivalenttothesamenumberofsingle-bondedatoms.
4.(R),(S)-Convention(IUPAC)
(1)Assignaprioritytoeachofthefouratomsorgroupsattachedtotheasymmetriccarbonatom.
(2)Thegroupoflowestpriorityisplacedinthepositionmostremotefromus.Theremaininggroupsareassignedpositionsinaclockwiseorderofdecreasingpriorityforthe(R)-isomerandinacounterclockwiseorderofdecreasingpriorityforthe(S)-isomer.
Fischer投影式:
将一手性碳原子的四面体球棍模型投影在纸面上得到的,投影时是假定把手性碳原子放在纸平面上,四面体的两个顶点,即两个原子或基团指向前方,用横线表示;四面体的另两个顶点,即另两个原子或基团指向后方,用竖线表示,通常简写成十字形。
一般总把含有碳原子的基团放在竖线相连的位置上。
对于Fischer投影式:
当手性碳原子连有四个不同基团时,可表示为C(a,b,c,d),按次序规则其优先次序假定为a>b>c>d,由于规定在竖线上的基团在纸平面的后面,若优先次序最小的集团d在竖线上,则符合上述观察条件,这时由a→b→c若是按顺时针方向排列,则手性碳原子构型为R构型,由a→b→c按逆时针方向排列,则为S构型。
若优先次序最小的基团d在横线上,则与上述观察条件相对,这时由a→b→c若是按顺时针方向排列,则手性碳原子构型为S构型,由a→b→c若按逆时针方向排列,则为R构型。
Enantiomersincycloalkanes
Enantiomersinconformationalformula
5.Diastereomers
Notecarefullythedifferencebetweenenantiomersanddiastereomers:
Enantiomershaveoppositeconfigurationsatallstereocenters;diastereomershaveoppositeconfigurationsatsome(oneormore)stereocentersbutthesameconfigurationatothers.
6.MesoCompounds.
The2R,3Sand2S,3Rstructuresareidenticalbecausethemoleculehasaplaneofsymmetryandisthereforeachiral.
BecauseoftheplaneofsymmetrythetartaricacidstereoisomershowninFigure6.11isachiral,despitethefactthatithastwostereocenters.Suchcompoundsthatareachiral,yetcontainstereocenters,arecalledmesocompounds
7.MoleculeswithMoreThanTwoStereocenters
Amoleculewithnstereocentershasamaximumof2nstereoisomers(2n-1pairsofenantiomers).
8.RacemicMixtures
Racemicmixturesareoftendenotedbythesymbol(±)orbytheprefixd,ltoindicatethattheycontainequalamountsofdextrorotatoryandlevorotatoryenantiomers.
9.Enantiomerswithnochiralcarbonatoms
ABriefReviewofIsomerism
10.Biologicaleffectsofchiralcompounds
interactionbetweenchiralmoleculeandbiologicalacceptor
Chapter7AlkylHalides
1.ClassificationandNomenclatureofhalohydrocarbon
Classification
Nomenclature
Commonname
Manysimplealkylhalidesarenamedbyidentifyingfirstthealkylgroupandthenthehalogen.
IUPACname
step1Findthelongestchain,andnameitastheparent.
step2Numberthecarbonsoftheparentchainbeginningattheendnearerthefirstsubstituent,regardlessofwhetheritisalkylorhalo.
3-bromo-4-methylhexane2-chloro-3,4-dimethyl-3-hexene
6-bromo-3-chloro-4-methylcyclohexene
3.Chemicalproperties
ThemoreimportantnucleophilicsubstitutionsofalkylhalidesareillustratedbythefollowingGereralequations
1.replacementofthehalogenatombyhydroxy(-OH)
reactivity:
R—I>R—Br>R—ClHydrolysisofalkylhalides
2.replacementofthehalogenatombycyano(-CN)
3.replacementofthehalogenatombyalkoxy(-OR)
4.Replacementofthehalogenatombyamino(-NH2)
5.Replacementofthehalogenatombymercapto(-SH)
6.Replacementofthehalogenatomby(-)
7.Replacementofthehalogenatomby-ONO2
Identificationofalkylhalides
Generalequation:
nucleophilic-substitution(SN)
nucleophilicreagent:
OH-、CN-、OR-、NH3、SH-、ONO2-
4.Mechanismofnucleophilicsubstitution.
SN1Mechanism
Formationofcarbocation
SN1EnergyDiagram
CharacteristicofSN1
•Unimolecularnucleophilicsubstitution.
•Twostepreactionwithcarbocationintermediate.
•Rateisfirstorderinthealkylhalide,zeroorderinthenucleophile.
•Racemizationoccurs.
StereochemistryofSN1Racemization:
inversionandretention
RatesofSN1Reactions
①3°>2°>1°>>CH3X
②Orderfollowsstabilityofcarbocations
③Morestableionrequireslessenergytoform
④Betterleavinggroup,fasterreaction
⑤Polarproticsolventbest:
Itsolvatesionsstronglywithhydrogenbonding.
SN2Mechanism
SN2EnergyDiagram
CharacteristicofSN2
•Bimolecularnucleophilicsubstitution.
•Concertedreaction:
newbondformingandoldbondbreakingatsametime.
•Rateisfirstorderineachreactant.
•Waldeninversion.
StereochemistryofSN2Waldeninversion
SN2:
ReactivityofSubstrate
①Carbonmustbepartiallypositive.
②Musthaveagoodleavinggroup.
③Carbonmustnotbestericallyhindered.
④CH3X>1°>2°>>3°TertiaryhalidesdonotreactviatheSN2mechanism,duetosterichindrance
5.FactorsaffectingSN1andSN2
(1)thestructureofthesubstrate.
(2)theeffectofthenucleophile(forSN2only).
(3)thenatureoftheleavinggroup.
(4)theeffectofthesolvent.
(1)thestructureofthesubstrate
Primaryormethyl(SN2)Tertiary(SN1)Secondary(SN2、SN1)
Allylicandbenzyl(SN2、SN1)
Vinylicandphenyl(noSN)
allylichalideandbenzylhalide
Vinylicandphenyl
(2)theeffectofthenucleophile(forSN2only).
Strongernucleophilesreactfaster.
Strongbasesarestrongnucleophiles,butnotallstrongnucleophilesarebasic
RS->CN->I->NH3(RNH2)>RO->OH->Br->CH3CO2->Cl->H2O>F-
(3)thenatureoftheleavinggroup.
①Electron-withdrawing
②Stableonceithasleft(notastrongbase)
Reactivity:
RI>RBr>RCl>RF
Badleavinggroup:
-OH,-OR,NH2-,CN-
(4)theeffectofthesolvent.
①Polarproticsolvents(O-HorN-H)reducethestrengthofthenucleophile.
②Hydrogenbondsmustbebrokenbeforenucleophilecanattackthecarbon.
③Polaraproticsolvents(noO-HorN-H)donotformhydrogenbondswithnucleophile
6.EliminationReactions
①Thealkylhalideloseshalogenasahalideion,andalsolosesH+ontheadjacentcarbontoabase.
②Apibondisformed.Productisalkene.
Alsocalleddehydrohalogenation(-HX).
examples
Saytzeff’sRule:
Ifmorethanoneeliminationproductispossible,themost-substitutedalkeneisthemajorproduct(moststable)
E1Reaction
•Unimolecularelimination
•Twogroupslost(usuallyX-andH+)
•Nucleophileactsasbase
•AlsohaveSN1products(mixture)
•Halideionleaves,formingcarbocation.
•BaseremovesH+fromadjacentcarbon.
•Pibondforms.
E2Re