Q1AR2中英文对照文档格式.docx
《Q1AR2中英文对照文档格式.docx》由会员分享,可在线阅读,更多相关《Q1AR2中英文对照文档格式.docx(38页珍藏版)》请在冰豆网上搜索。
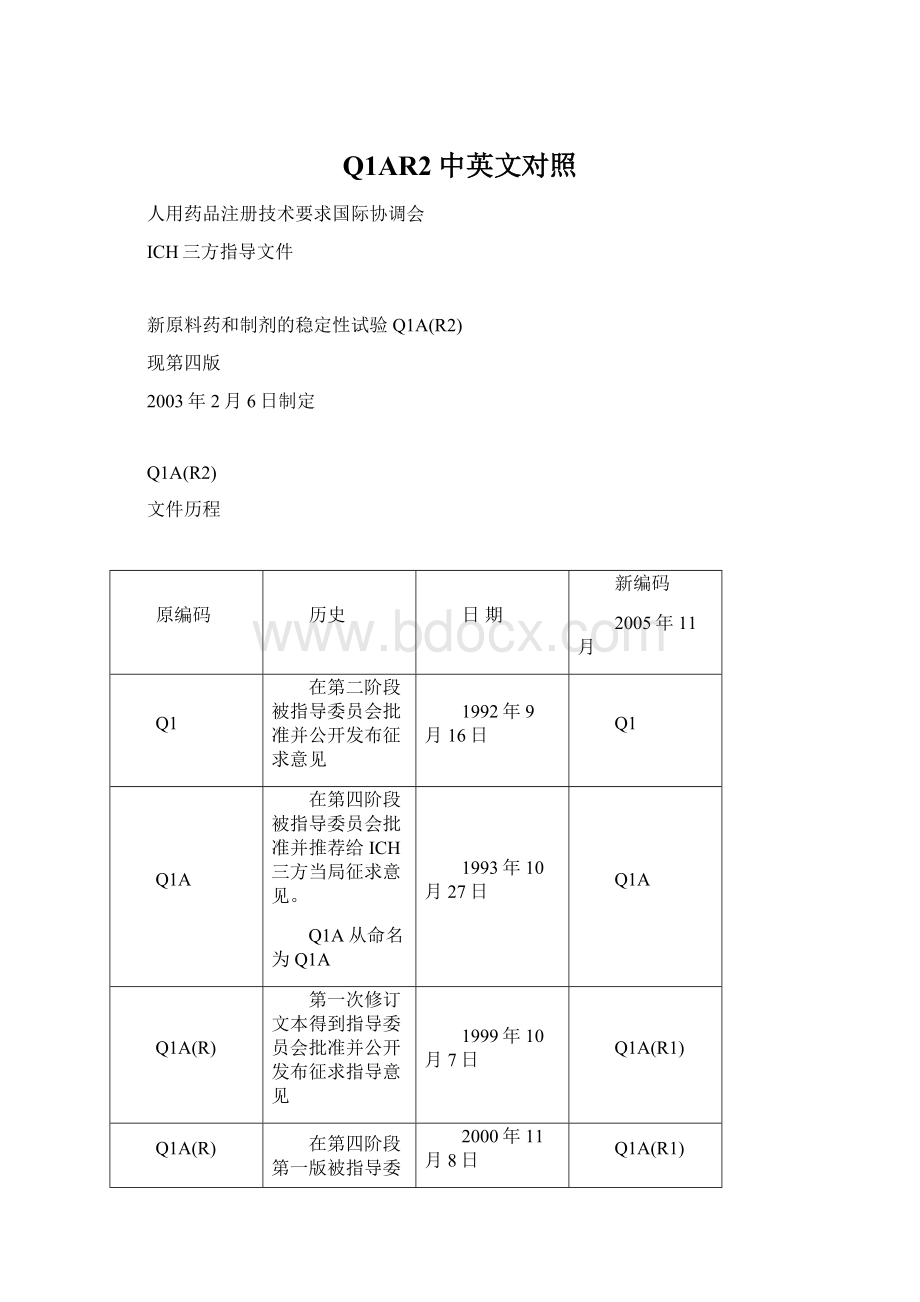
新编码
2005年11月
Q1
在第二阶段被指导委员会批准并公开发布征求意见
1992年9月16日
Q1A
在第四阶段被指导委员会批准并推荐给ICH三方当局征求意见。
Q1A从命名为Q1A
1993年10月27日
Q1A(R)
第一次修订文本得到指导委员会批准并公开发布征求指导意见
1999年10月7日
Q1A(R1)
在第四阶段第一版被指导委员会批准并推荐给ICH三方当局征求意见。
2000年11月8日
第四阶段第二版直接被指导委员会批准,没有公开发布征求意见。
此文本包含了因采纳Q1F(Ⅲ和Ⅳ气候带注册申请的稳定性数据包)所引起的改变,并推荐给ICH三方当局采纳。
2003年2月6日
新原料药和制剂的稳定性试验Q1A(R)修订说明
本修订的目的为了明确由于采用了ICHQ1F“在气候带Ⅲ和Ⅳ注册申请的稳定性数据包”而使Q1A(R)而产生的变更。
这些变更如下:
1.在下面章节中将中间储存条件从温度30℃±
2℃/相对湿度60%±
5%修改为温度30℃±
2℃/相对湿度65%±
5%:
2.1.7.1原料药-储存条件-一般情况
2.2.7.1制剂-储存条件-一般情况
2.2.7.3在半渗透性容器中包装的制剂
3术语-“中间试验”
2.在下面章节中可以使用温度30℃±
5%替代温度25℃±
5%作为长期稳定性试验的条件:
3.在温度25℃±
2℃/相对湿度40%±
5%的基础上增加了温度30℃±
2℃/相对湿度35%±
5%作为长期稳定性试验条件,并且在后面的章节中包括了失水比率相关举例的相关情况:
2.2.7.3在半透性容器中包装的制剂
在试验阶段中间将中间将储存条件从温度30℃±
5%调整为温度30℃±
5%是可以的,但相应的储存条件和调整的日期要在注册申报资料中清楚地说明和列出。
如果适用的话建议ICH三方在公布和执行此修订指南三年后,注册申请资料中完整的试验能够包含在中间储存条件,即温度30℃±
5%下的实验资料。
STABILITYTESTINGOFNEWDRUGSUBSTANCESANDPRODUCTS
1.INTRODUCTION
1.1.ObjectivesoftheGuideline
ThefollowingguidelineisarevisedversionoftheICHQ1AguidelineanddefinesthestabilitydatapackageforanewdrugsubstanceordrugproductthatissufficientforaregistrationapplicationwithinthethreeregionsoftheEC,Japan,andtheUnitedStates.Itdoesnotseeknecessarilytocoverthetestingforregistrationinorexporttootherareasoftheworld.
Theguidelineseekstoexemplifythecorestabilitydatapackagefornewdrugsubstancesandproducts,butleavessufficientflexibilitytoencompassthevarietyofdifferentpracticalsituationsthatmaybeencounteredduetospecificscientificconsiderationsandcharacteristicsofthematerialsbeingevaluated.Alternativeapproachescanbeusedwhentherearescientificallyjustifiablereasons.
1.2.ScopeoftheGuideline
Theguidelineaddressestheinformationtobesubmittedinregistrationapplicationsfornewmolecularentitiesandassociateddrugproducts.Thisguidelinedoesnotcurrentlyseektocovertheinformationtobesubmittedforabbreviatedorabridgedapplications,variations,clinicaltrialapplications,etc.
Specificdetailsofthesamplingandtestingforparticulardosageformsintheirproposedcontainerclosuresarenotcoveredinthisguideline.
Furtherguidanceonnewdosageformsandonbiotechnological/biologicalproductscanbefoundinICHguidelinesQ1CandQ5C,respectively.
1.3.GeneralPrinciples
Thepurposeofstabilitytestingistoprovideevidenceonhowthequalityofadrugsubstanceordrugproductvarieswithtimeundertheinfluenceofavarietyofenvironmentalfactorssuchastemperature,humidity,andlight,andtoestablishare-testperiodforthedrugsubstanceorashelflifeforthedrugproductandrecommendedstorageconditions.
ThechoiceoftestconditionsdefinedinthisguidelineisbasedonananalysisoftheeffectsofclimaticconditionsinthethreeregionsoftheEC,JapanandtheUnitedStates.Themeankinetictemperatureinanypartoftheworldcanbederivedfromclimaticdata,andtheworldcanbedividedintofourclimaticzones,I-IV.ThisguidelineaddressesclimaticzonesIandII.TheprinciplehasbeenestablishedthatstabilityinformationgeneratedinanyoneofthethreeregionsoftheEC,JapanandtheUnitedStateswouldbemutuallyacceptabletotheothertworegions,providedtheinformationisconsistentwiththisguidelineandthelabelingisinaccordwithnational/regionalrequirements.
2.GUIDELINES
2.1.DrugSubstance
2.1.1.General
Informationonthestabilityofthedrugsubstanceisanintegralpartofthesystematicapproachtostabilityevaluation.
2.1.2.StressTesting
Stresstestingofthedrugsubstancecanhelpidentifythelikelydegradationproducts,whichcaninturnhelpestablishthedegradationpathwaysandtheintrinsicstabilityofthemoleculeandvalidatethestabilityindicatingpoweroftheanalyticalproceduresused.Thenatureofthestresstestingwilldependontheindividualdrugsubstanceandthetypeofdrugproductinvolved.
Stresstestingislikelytobecarriedoutonasinglebatchofthedrugsubstance.Itshouldincludetheeffectoftemperatures(in10°
Cincrements(e.g.,50°
C,60°
C,etc.)abovethatforacceleratedtesting),humidity(e.g.,75%RHorgreater)whereappropriate,oxidation,andphotolysisonthedrugsubstance.ThetestingshouldalsoevaluatethesusceptibilityofthedrugsubstancetohydrolysisacrossawiderangeofpHvalueswheninsolutionorsuspension.Photostabilitytestingshouldbeanintegralpartofstresstesting.ThestandardconditionsforphotostabilitytestingaredescribedinICHQ1B.
Examiningdegradationproductsunderstressconditionsisusefulinestablishingdegradationpathwaysanddevelopingandvalidatingsuitableanalyticalprocedures.However,itmaynotbenecessarytoexaminespecificallyforcertaindegradationproductsifithasbeendemonstratedthattheyarenotformedunderacceleratedorlongtermstorageconditions.
Resultsfromthesestudieswillformanintegralpartoftheinformationprovidedtoregulatoryauthorities.
2.1.3.SelectionofBatches
Datafromformalstabilitystudiesshouldbeprovidedonatleastthreeprimarybatchesofthedrugsubstance.Thebatchesshouldbemanufacturedtoaminimumofpilotscalebythesamesyntheticrouteas,andusingamethodofmanufactureandprocedurethatsimulatesthefinalprocesstobeusedfor,productionbatches.Theoverallqualityofthebatchesofdrugsubstanceplacedonformalstabilitystudiesshouldberepresentativeofthequalityofthematerialtobemadeonaproductionscale.
Othersupportingdatacanbeprovided.
2.1.4.ContainerClosureSystem
Thestabilitystudiesshouldbeconductedonthedrugsubstancepackagedinacontainerclosuresystemthatisthesameasorsimulatesthepackagingproposedforstorageanddistribution.
2.1.5.Specification
Specification,whichisalistoftests,referencetoanalyticalprocedures,andproposedacceptancecriteria,isaddressedinICHQ6AandQ6B.Inaddition,specificationfordegradationproductsinadrugsubstanceisdiscussedinQ3A.
Stabilitystudiesshouldincludetestingofthoseattributesofthedrugsubstancethataresusceptibletochangeduringstorageandarelikelytoinfluencequality,safety,and/orefficacy.Thetestingshouldcover,asappropriate,thephysical,chemical,biological,andmicrobiologicalattributes.Validatedstability-indicatinganalyticalproceduresshouldbeapplied.Whetherandtowhatextentreplicationshouldbeperformedwilldependontheresultsfromvalidationstudies.
2.1.6.TestingFrequency
Forlongtermstudies,frequencyoftestingshouldbesufficienttoestablishthestabilityprofileofthedrugsubstance.Fordrugsubstanceswithaproposedre-testperiodofatleast12months,thefrequencyoftestingatthelongtermstorageconditionshouldnormallybeevery3monthsoverthefirstyear,every6monthsoverthesecondyear,andannuallythereafterthroughtheproposedre-testperiod.
Attheacceleratedstoragecondition,aminimumofthreetimepoints,includingtheinitialandfinaltimepoints(e.g.,0,3,and6months),froma6-monthstudyisrecommended.Whereanexpectation(basedondevelopmentexperience)existsthatresultsfromacceleratedstudiesarelikelytoapproachsignificantchangecriteria,increasedtestingshouldbeconductedeitherbyaddingsamplesatthefinaltimepointorbyincludingafourthtimepointinthestudydesign.
Whentestingattheintermediatestorageconditioniscalledforasaresultofsignificantchangeattheacceleratedstoragecondition,aminimumoffourtimepoints,includingtheinitialandfinaltimepoints(e.g.,0,6,9,12months),froma12-monthstudyisrecommended.
2.1.7.StorageConditions
Ingeneral,adrugsubstanceshouldbeevaluatedunderstorageconditions(withappropriatetolerances)thattestitsthermalstabilityand,ifapplicable,itssensitivitytomoisture.Thestorageconditionsandthelengthsofstudieschosenshouldbesufficienttocoverstorage,shipment,andsubsequentuse.
Thelongtermtestingshouldcoveraminimumof12months’durationonatleastthreeprimarybatchesatthetimeofsubmissionandshouldbecontinuedforaperiodoftimesufficienttocovertheproposedre-testperiod.Additionaldataaccumulatedduringtheassessmentperiodoftheregistrationapplicationshouldbesubmittedtotheauthoritiesifrequested.Datafromtheacceleratedstorageconditionand,ifappropriate,fromtheintermediatestorageconditioncanbeusedtoevaluatetheeffectofshorttermexcursionsoutsidethelabelstorageconditions(suchasmightoccurduringshipping).
Longterm,accelerated,and,whereappropriate,intermediatestorageconditionsfordrugsubstancesaredetailedinthesectionsbelow.Thegeneralcaseappliesifthedrugsubstanceisnotspecificallycoveredbyasubsequentsection.Alternativestorageconditionscanbeusedifjustified.
2.1.7.1.Generalcase
Study
Storagecondition
Minimumtimeperiodcoveredbydataatsubmission
Longterm*
25°
C±
2°
C/60%RH±
5%RHor30°
C/65%RH±
5%RH
12months
Intermediate**
30°
6months
Accelerated
40°
C/75%RH±
6months
*Itisuptotheapplicanttodecidewhetherlongtermstabilitystudiesareperformedat25±
2°
C±
5%RH.
**If30°
5%RHisthelong-termcondition,thereisnointermediatecondition.
Iflong-termstudiesareconductedat25°
C/60%RH