重新包装管理规程完整.docx
《重新包装管理规程完整.docx》由会员分享,可在线阅读,更多相关《重新包装管理规程完整.docx(31页珍藏版)》请在冰豆网上搜索。
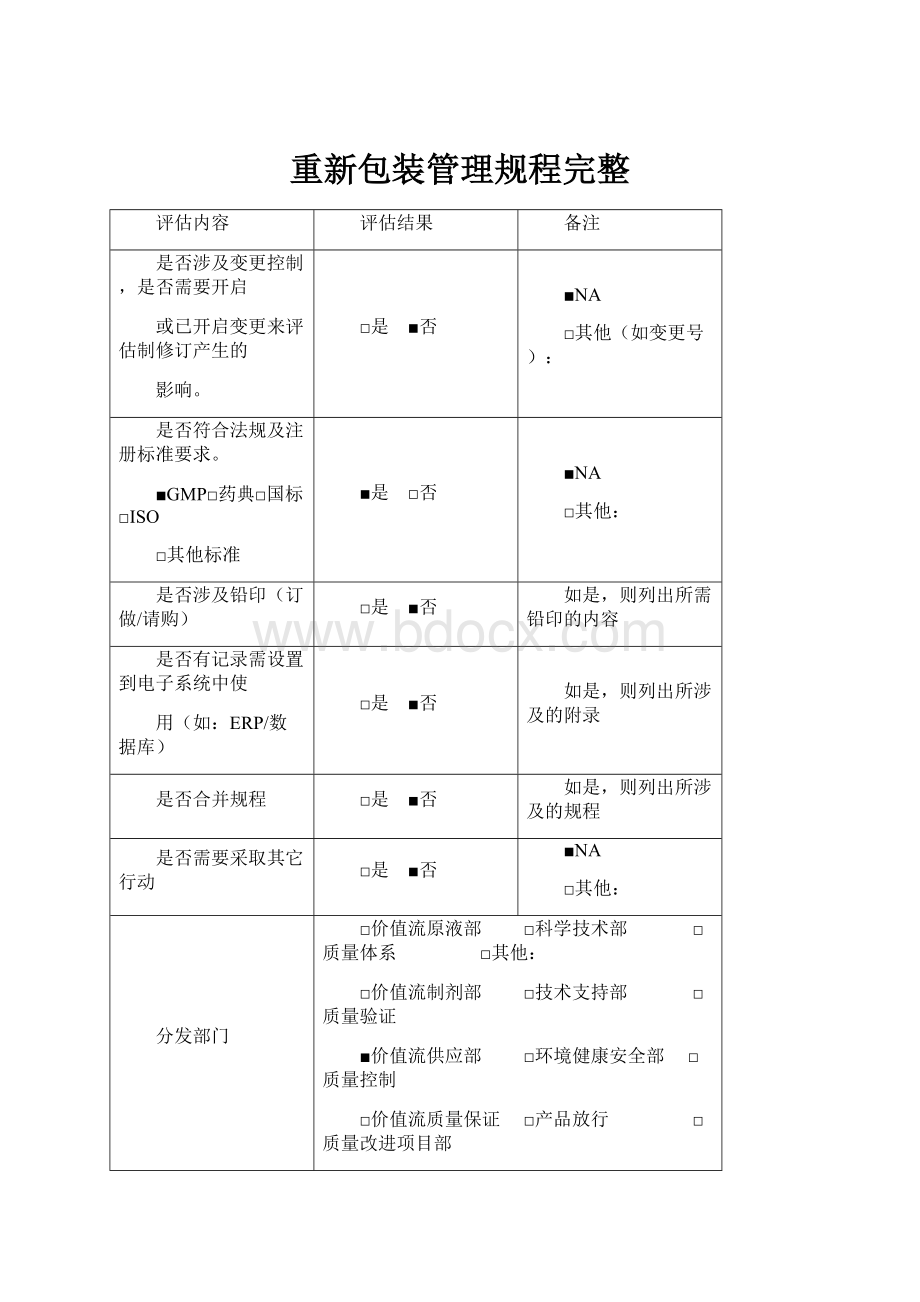
重新包装管理规程完整
评估内容
评估结果
备注
是否涉及变更控制,是否需要开启
或已开启变更来评估制修订产生的
影响。
□是 ■否
■NA
□其他(如变更号):
是否符合法规及注册标准要求。
■GMP□药典□国标□ISO
□其他标准
■是 □否
■NA
□其他:
是否涉及铅印(订做/请购)
□是 ■否
如是,则列出所需铅印的内容
是否有记录需设置到电子系统中使
用(如:
ERP/数据库)
□是 ■否
如是,则列出所涉及的附录
是否合并规程
□是 ■否
如是,则列出所涉及的规程
是否需要采取其它行动
□是 ■否
■NA
□其他:
分发部门
□价值流原液部 □科学技术部 □质量体系 □其他:
□价值流制剂部 □技术支持部 □质量验证
■价值流供应部 □环境健康安全部 □质量控制
□价值流质量保证 □产品放行 □质量改进项目部
规程编号/版本号
Number/Version
SMP.G.00.PR.028/06
制/修订部门
Department
QAVS
规程页码
Page of Pages
1 / 15
规程名称
Title
产品重新包装管理规程 Product Repackaging Management
规程编号/版本号
Number/Version
SMP.G.00.PR.028/06
制/修订部门
Department
QAVS
规程页码
Page of Pages
2 / 15
规程名称
Title
产品重新包装管理规程 Product Repackaging Management
第一部分 正文
1.目的∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 3 页
2.适用范围∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 3 页
3.责任部门(人) ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 4-5 页
4.定义和缩略语 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 5-6 页
5.物料和设备∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 6 页
6.规程 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 6-12 页
7.附件∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 12 页
8.附录∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 12 页
9.参考文件 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 12-13 页
10.注意事项∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 13 页
第二部分 正文附件
附件 A ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙第 14-15 页
重新包装种类 Types of Repacking
适用于 Applies To
不适用于 Does Not Apply To
所有第二次包装操作均发生在成品已经过QP放行或
其他同等批次放行流程放行后,和/或该产品已离开
原始生产地后,具体内容包括:
All Secondary Packing Operations that take place after
the finished product has been released following QP
certification or equivalent batch release process and/or
has left the original manufacturing site, including:
✓ 外包装材料的变更,添加,替代和/或去除
Alteration, addition, substitution and/or removal of
secondary packaging
✓ 组分(例如:
说明书,内盒)Components (For
example:
leaflets, cartons).
✓ 添加防篡改或防伪标志Addition of tamper
evident or ant counterfeit features.
禁止在西林瓶,安瓿瓶,注射器及任何疫苗产品的初次
包装上打印或覆盖贴标。
任何重新包装发生在成品放行
之前和/或离开原始生产地之前,具体内容包括:
Printing or over-labeling on vials, ampoules, syringes and any
vaccines product primary packaging as this is prohibited.
Any repacking, which takes place before the finished product
has been released and/or has left the original manufacturing
site, This includes:
o 任何工序涉及内包装材料破坏(或无菌生物制品包
装开口/破损)Any process that involves the breaking of
the primary pack (or blister opening/breaching for sterile
and biological products).
o 质量许可情况下,在某一工厂对半成品做进一步处
理(贴标或包装)Semi-finished product further
processed by a site (labeling and packaging) and for
which the activity is covered in Quality Agreement.
规程编号/版本号
Number/Version
SMP.G.00.PR.028/06
制/修订部门
Department
QAVS
规程页码
Page of Pages
3 / 15
规程名称
Title
产品重新包装管理规程 Product Repackaging Management
The table below details the types of repacking to which the process does or does not apply.:
●产品重新包装管理规程 Product Reprocessing and Repackaging Management
1. 目的 Objective / Purpose
1.1 明确返工和重新包装的定义 Definition of Reprocessing and Repacking
1.2明确返工要求和重新包装操作的管理流程、职责 Define Reprocessing requirement and the process and
responsibility of Repackaging.
2. 适用范围 Scope
2.1 重新包装范围见下表 1:
The scope of Repackaging is listed in table 1 below:
2.2TY 所有的商业产品均不得进行返工、重新加工;Reprocessing and reworking is not allowed for all
commercial product in Tianyuan manufacturing site.
工艺过程中适用与不适用重新包装的类型详见下表1:
商业角色Business Role
关键职责Key Responsibilities
重新包装发起人
Repacking Proposer
评估重新包装,确保评估是基于商业,监管,市场及产品质量要求。
To assess the
repacking activity and ensure it is justified based on commercial, regulatory, market and
product quality requirements.
评估报告在重新包装开始前得到批准To obtain appropriate approval for the
repacking before it starts.
确保重新包装活动是有记录追踪的。
To ensure that repacking activity is captured in
a local tracker.
规程编号/版本号
Number/Version
SMP.G.00.PR.028/06
制/修订部门
Department
QAVS
规程页码
Page of Pages
4 / 15
规程名称
Title
产品重新包装管理规程 Product Repackaging Management
approvals is maintained.
✓由于产品需从一个市场转移到其他市场,重新
包装或重新贴标签以符合当地语言要求。
Repacking or relabeling to meet local language
requirements as a result of transfer of product form
one market to anther market
✓初次包装后已放行到市场Packaging of primary
o 产品放行上市之前在原生产地进行的成品重新包装
Repacking of finished packs at original packing site
before the product is released for sale.
o 在产品内盒外添加VVM标签Add the VVM labeling on
the carton.
packs released to the market.。
3. 责任部门(人)Responsible department (person)
关键的角色和职责KEY ROLES AND RESPONSIBILITIES
以下个人和团队有重要的角色和职责来完成重新包装流程。
同一个人可能担任不止一个角色,保留必要的独
立批准职责 The following individuals and teams have key roles and responsibilities to fulfill in the Repacking
process. The same individual may perform more than one role, provided the necessary independence of required
质量部-Quality QAVS
确保重新包装的流程是有效的和健全的,重新包装符合该SMP要求。
To ensure
effective and robust processes are in place and in use to manage repacking, in accordance
with this SMP.
审阅和批准重新包装申请 To review and approve repacking proposals.
在重新包装开始之前获得适当额外的批准 To obtain appropriate additional
approvals for the repacking before it starts.
确保重新包装的设施能满足用途,例如符合要求,文件,设备及人员充足 To
ensure that repacking facilities are fit for purpose, i.e. comply with requirements and
adequate documentation, equipment and personnel are in place.
确保任何发起的重新包装的法规影响经过评估和记录,否则委托给重新包装者或
原始制造地(必要时QS支持)
To ensure that any regulatory implications of any proposed repacking have been assessed
and addressed. Unless delegated to the repacker or original manufacturing site.
批准或拒绝与药品相关的重新包装的要求 To approve or reject repacking requests
related to medicinal products.
放行QA/QP
QP/QA Release
审阅和批准重新包装申请(二级)To review and approve repacking proposals.
确保所有重新包装的组分是完全受控的 To ensure all repacking components are
adequately controlled.
在年度APR中回顾重新包装的批次 To periodically review repacking batch in the APR
Report .
放行重新包装的材料(放行QA) To release components for repacking (QA Release)
批准或拒绝与药品相关的重新包装的要求(二级)To approve or reject repacking
requests related to medicinal products .
按照法规要求放行重新包装产品 To release repacked products where required by
regulations.
仓库管理员 Warehouse
Handler
把所重新检查和重新包装的批号分开存放 Segregate physically the recheck and
repack batches and indicate in the inventory logbook..
规程编号/版本号
Number/Version
SMP.G.00.PR.028/06
制/修订部门
Department
QAVS
规程页码
Page of Pages
5 / 15
规程名称
Title
产品重新包装管理规程 Product Repackaging Management
规程编号/版本号
Number/Version
SMP.G.00.PR.028/06
制/修订部门
Department
QAVS
规程页码
Page of Pages
6 / 15
规程名称
Title
产品重新包装管理规程 Product Repackaging Management
4. 定义和缩略语 Definition and abbreviation
重新包装:
任何内包装材料或者外包装材料的文字的更改,,或去除或替换任何已打印外包装材料,并且操作
均发生在成品已按照QP放行或其他同等批次放行流程放行后,和/或该产品已离开原始生产地后。
Any operation that:
Requires the alteration of text to primary or secondary packaging and/or the addition, removal or exchange of any
printed secondary packaging material, and takes place after the finished product has been released following QP
certification or equivalent batch release process and/or has left the original manufacturing site.
5. 物料和设备 Materials and equipment
N/A
6. 规程 Procedure
6.1 重新包装
6.1.1重新包装评估(参见附录1)Assessment of Repacking
重新包装申请者必须评估重新包装开始之前所有行动是否合理,要求:
The Repacking Proposer must assess if
repacking is justified before starting any repacking activities. They must:
6.1.1.1评估重新包装的理由 Evaluate the reason(s) why repacking is required.
由于偏差而需要重新包装时,确认必要的纠正预防措施已经完成 When required because of a deviation, confirm
that necessary corrective and preventative actions (CAPAs) are complete.
6.1.1.2评估申请的重新包装的商业可行性,需考虑重新包装批次的成本,供应的重要性及再包装产品剩余保质
期 Evaluate commercial viability of proposed repacking, taking into account total cost, criticality of supply and extent
of shelf life remaining for any repacked batches.
6.1.1.3定义范围,比如包装需要改变,包装的数目 Identify what would be involved, for example changes to be
made to the pack, number of packs to be processed.
6.1.1.4评估在什么地方进行重新包装操作,确认以下内容被批准 Evaluate where the repacking operations would
be conducted and confirm that the following are in place and approved:
A:
供应的技术项目 Technical terms of supply .
B:
对于第三方,第三方操作现场审计,技术/质量方协议和合同 For third parties, Site audit for proposed
activity, technical/quality agreements and contracts.
6.1.1.5识别待供应的市场,法规及批次放行的影响 Identify markets to be supplied, regulatory and batch release