Paracetamol扑热息痛.docx
《Paracetamol扑热息痛.docx》由会员分享,可在线阅读,更多相关《Paracetamol扑热息痛.docx(18页珍藏版)》请在冰豆网上搜索。
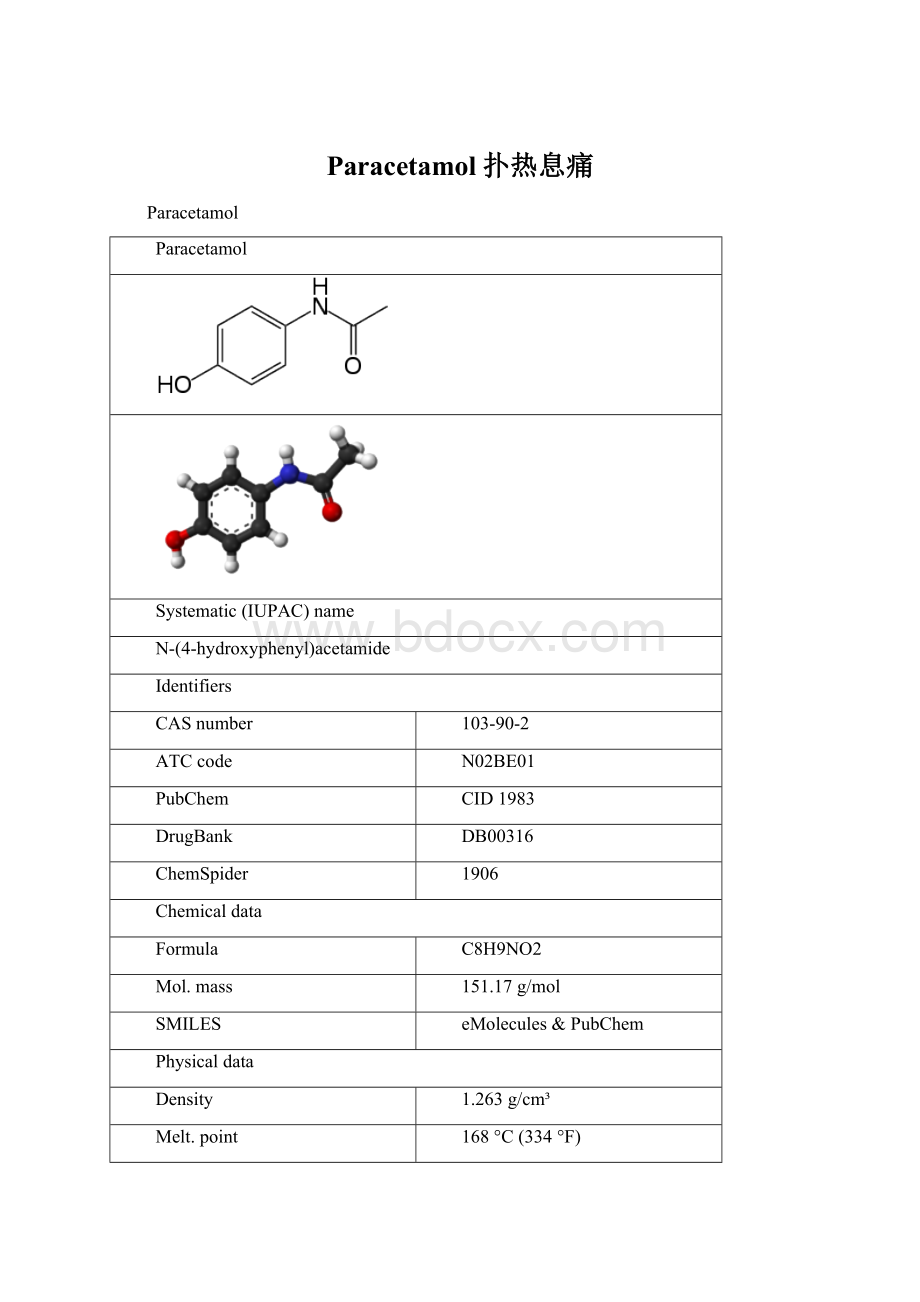
Paracetamol扑热息痛
Paracetamol
Paracetamol
Systematic(IUPAC)name
N-(4-hydroxyphenyl)acetamide
Identifiers
CASnumber
103-90-2
ATCcode
N02BE01
PubChem
CID1983
DrugBank
DB00316
ChemSpider
1906
Chemicaldata
Formula
C8H9NO2
Mol.mass
151.17g/mol
SMILES
eMolecules & PubChem
Physicaldata
Density
1.263 g/cm³
Melt.point
168 °C(334 °F)
Solubility inwater
12.78 [1] mg/mL(20 °C)
Pharmacokineticdata
Bioavailability
~100%
Metabolism
90to95% Hepatic
Half-life
1–4h
Excretion
Renal
Therapeuticconsiderations
Licencedata
USFDA:
link
Pregnancycat.
A(AU) B(US) safe
Legalstatus
Unscheduled (AU) GSL (UK) OTC(US)
Routes
Oral, rectal, intravenous
(whatisthis?
) (verify)
Paracetamol (internationalnonproprietaryname)(pronounced /ˌpærəˈsiːtəmɒl,ˌpærəˈsɛtəmɒl/)or acetaminophen (/əˌsiːtəˈmɪnɵfɨn/ (
listen))(USAN)isawidelyused over-the-counter analgesic (painreliever)and antipyretic (feverreducer).
Itiscommonlyusedforthereliefof headaches,andotherminorachesandpains,andisamajoringredientinnumerous cold and flu remedies.Incombinationwith opioidanalgesics,paracetamolcanalsobeusedinthemanagementofmoreseverepainsuchaspostsurgicalpainandprovidingpalliativecareinadvancedcancerpatients.[2] Theonsetofanalgesiaisapproximately11minutesafter oraladministration ofparacetamol,[3] andits halflifeis1–4hours.
Whilegenerallysafeforuseatrecommendeddoses(1,000mgpersingledose andupto 4,000mgperday foradults,upto 2,000mgperday ifdrinkingalcohol),[4] acute overdoses ofparacetamolcancausepotentiallyfatal liverdamage and,inrareindividuals,anormaldosecandothesame;theriskisheightenedby alcoholconsumption. Paracetamoltoxicity istheforemostcauseof acuteliverfailure inthe Westernworld,andaccountsformostdrugoverdosesintheUnitedStates,theUnitedKingdom,AustraliaandNewZealand.[5][6][7][8]
Paracetamolispartoftheclassofdrugsknownas"aniline analgesics";itistheonlysuchdrugstillinusetoday.[9] Itistheactivemetaboliteof phenacetin,oncepopularasananalgesicandantipyreticinitsownright,butunlikephenacetinanditscombinations,paracetamolisnotconsideredtobe carcinogenicattherapeuticdoses.[10] Thewords acetaminophen (usedintheUnitedStates,Canada,HongKong,Iran,[11] ColombiaandotherLatinAmericancountries)and paracetamol (usedelsewhere)bothcomefromchemicalnamesforthecompound:
para-acetylaminophenoland para-acetylaminophenol.Insomecontexts,itissimplyabbreviatedas APAP,for N-acetyl-para-aminophenol.
Theclassificationofparacetamol,andtheterminologyusedtorefertoit,cancauseconfusion.Itisoftenclassifiedasanonsteroidalanti-inflammatorydrug(NSAID),butparacetamolhasfewanti-inflammatoryeffectsinmanytissues.However, aspirin,paracetamolandotherNSAIDsallactbythesamemechanism(inhibitionof prostaglandin synthesis)andallshowvaryinglevelsofanalgesic,anti-inflammatory,antipyreticandantiplateletactions.[12]
Contents
[hide]
∙1 History
∙2 Structureandreactivity
∙3 Synthesis
∙4 Reactions
∙5 Availableforms
∙6 Mechanismofaction
∙7 Metabolism
∙8 Indications
∙9 Efficacyandsideeffects
o9.1 Efficacy
o9.2 Adverseeffects
∙10 Toxicity
∙11 Effectsonanimals
∙12 References
∙13 Externallinks
[edit]History
Acetanilide wasthefirstanilinederivativeserendipitouslyfoundtopossessanalgesicaswellasantipyreticproperties,andwasquicklyintroducedintomedicalpracticeunderthenameof Antifebrin byA.CahnandP.Heppin1886.[13] Butitsunacceptabletoxiceffects,themostalarmingbeing cyanosis dueto methemoglobinemia,promptedthesearchforlesstoxicanilinederivatives.[9] HarmonNorthropMorse hadalreadysynthesizedparacetamolat JohnsHopkinsUniversity viathereductionof p-nitrophenol with tin inglacial aceticacid in1877,[14][15] butitwasnotuntil1887thatclinicalpharmacologist JosephvonMering triedparacetamolonpatients.[9] In1893,vonMeringpublishedapaperreportingontheclinicalresultsofparacetamolwith phenacetin,anotheranilinederivative.[16] VonMeringclaimedthat,unlikephenacetin,paracetamolhadaslighttendencytoproducemethemoglobinemia.Paracetamolwasthenquicklydiscardedinfavorofphenacetin.Thesalesofphenacetinestablished Bayer asaleadingpharmaceuticalcompany.[17] Overshadowedinpartbyaspirin,introducedintomedicineby HeinrichDreser in1899,phenacetinwaspopularformanydecades,particularlyinwidelyadvertisedover-the-counter"headachemixtures,"usuallycontainingphenacetin,an aminopyrine derivativeofaspirin,caffeine,andsometimesa barbiturate.[9]
VonMering'sclaimsremainedessentiallyunchallengedforhalfacentury,untiltwoteamsofresearchersfromtheUnitedStatesanalyzedthemetabolismofacetanilideandparacetamol.[17] In1947DavidLester andLeonGreenbergfoundstrongevidencethatparacetamolwasamajormetaboliteofacetanilideinhumanblood,andinasubsequentstudytheyreportedthatlargedosesofparacetamolgiventoalbinoratsdidnotcausemethemoglobinemia.[18] InthreepaperspublishedintheSeptember1948issueofthe JournalofPharmacologyandExperimentalTherapeutics, BernardBrodie, JuliusAxelrod andFrederickFlinnconfirmedusingmorespecificmethodsthatparacetamolwasthemajormetaboliteofacetanilideinhumanblood,andestablisheditwasjustasefficaciousananalgesicasitsprecursor.[19][20][21] Theyalsosuggestedthatmethemoglobinemiaisproducedinhumansmainlybyanothermetabolite, phenylhydroxylamine.AfollowuppaperbyBrodieandAxelrodin1949establishedthatphenacetinwasalsometabolizedtoparacetamol.[22] Thisledtoa"rediscovery"ofparacetamol.[9] Ithasbeensuggestedthatcontaminationofparacetamolwith 4-aminophenol,thesubstancefromwhichitwassynthesizedbyvonMering,maybethecauseforhisspuriousfindings.[17]
BernardBrodie and JuliusAxelrod(pictured) demonstratedthatacetanilideandphenacetinarebothmetabolizedtoparacetamol,whichisabettertoleratedanalgesic.
ParacetamolwasfirstmarketedintheUnitedStatesin1953by Sterling-WinthropCo.,whichpromoteditaspreferabletoaspirinsinceitwassafetotakeforchildrenandpeoplewithulcers.[17] ThebestknownbrandtodayforparacetamolintheUnitedStates, Tylenol,wasestablishedin1955when McNeilLaboratoriesstartedsellingparacetamolasapainandfeverrelieverforchildren,underthebrandnameTylenolChildren'sElixir—theword"tylenol"wasacontractionof para-acetylaminophenol.[23] In1956,500 mg tabletsofparacetamolwentonsaleintheUnitedKingdomunderthetradenamePanadol,producedbyFrederickStearns&Co,asubsidiaryof SterlingDrug Inc.Panadolwasoriginallyavailableonlybyprescription,forthereliefofpainandfever,andwasadvertisedasbeing"gentletothestomach,"sinceotheranalgesicagentsofthetimecontainedaspirin,aknownstomachirritant.[citationneeded] In1963,paracetamolwasaddedtothe BritishPharmacopoeia,andhasgainedpopularitysincethenasananalgesicagentwithfewside-effectsandlittleinteractionwithotherpharmaceuticalagents.[15] Concernsaboutparacetamol'ssafetydelayeditswidespreadacceptanceuntilthe1970s,butinthe1980sparacetamolsalesexceededthoseofaspirininmanycountries,includingtheUnitedKingdom.Thiswasaccompaniedbythecommercialdemiseofphenacetin,blamedasthecauseof analgesicnephropathy andhematologicaltoxicity.[9]
TheU.S. patent onparacetamolhaslongexpired,andgenericversionsofthedrugarewidelyavailableunderthe DrugPriceCompetitionandPatentTermRestorationAct of1984,althoughcertainTylenolpreparationswereprotecteduntil2007.U.S.patent6,126,967filedSeptember3,1998wasgrantedfor"Extendedreleaseacetaminophenparticles".[24]
[edit]Structureandreactivity
PSA oftheparacetamolmolecule
Paracetamolconsistsofa benzene ringcore, substituted byone hydroxyl groupandthe nitrogen atomofan amide groupinthe para (1,4) pattern.[25] Theamidegroupis acetamide (ethanamide).Itisanextensively conjugatedsystem,asthe lonepair onthehydroxyloxygen,thebenzenepicloud,thenitrogenlonepair,the porbital onthe carbonyl carbon,andthelonepaironthecarbonyloxygenareallconjugated.Thepresenceoftwoactivatinggroupsalsomakethebenzeneringhighlyreactivetoward electrophilic aromaticsubstitution.Asthesubstituentsareortho,para-directingandparawithrespecttoeachother,allpositionsontheringaremoreorlessequallyactivated.Theconjugationalsogreatlyreducesthe basicity oftheoxygensandthenitrogen,whilemakingthehydroxylacidicthroughdelocalisationofchargedevelopedonthe phenoxide anion.
[edit]Synthesis
Comparedwithmanyotherdrugs,paracetamolismucheasiertosynthesize,becauseitlacks stereocenters.Asaresult,thereisnoneedtodesignastereo-selectivesynthesis.
Industrialpreparationofparacetamolusuallyproceedsfrom nitrobenzene.[26] Aone-stepreductiveacetamidationreactioncanbemediatedbythioacetate.[27]
Paracetamolmaybeeasilypreparedinthelaboratoryby nitrating phenolwith sodiumnitrate,separatingthedesired p-nitrophenol fromthe ortho-byproduct,andreducingthe nitrogroup with sodiumborohydride.Theresultant p-aminophenol isthenacetylatedwith aceticanhydride.[28] Inthisreaction, phenol isstronglyactivating,thusthereactionrequiresonlymildconditions(cf.thenitrationofbenzene):
[edit]Reactions
p-Aminophenol maybeobtainedbytheamide hydrolysis ofparacetamol. p-Aminophenolpreparedthisway,andrelatedtothecommerciallyavailable Metol,hasbeenusedasa