31251484Chapter6Electrochemistry.docx
《31251484Chapter6Electrochemistry.docx》由会员分享,可在线阅读,更多相关《31251484Chapter6Electrochemistry.docx(12页珍藏版)》请在冰豆网上搜索。
31251484Chapter6Electrochemistry
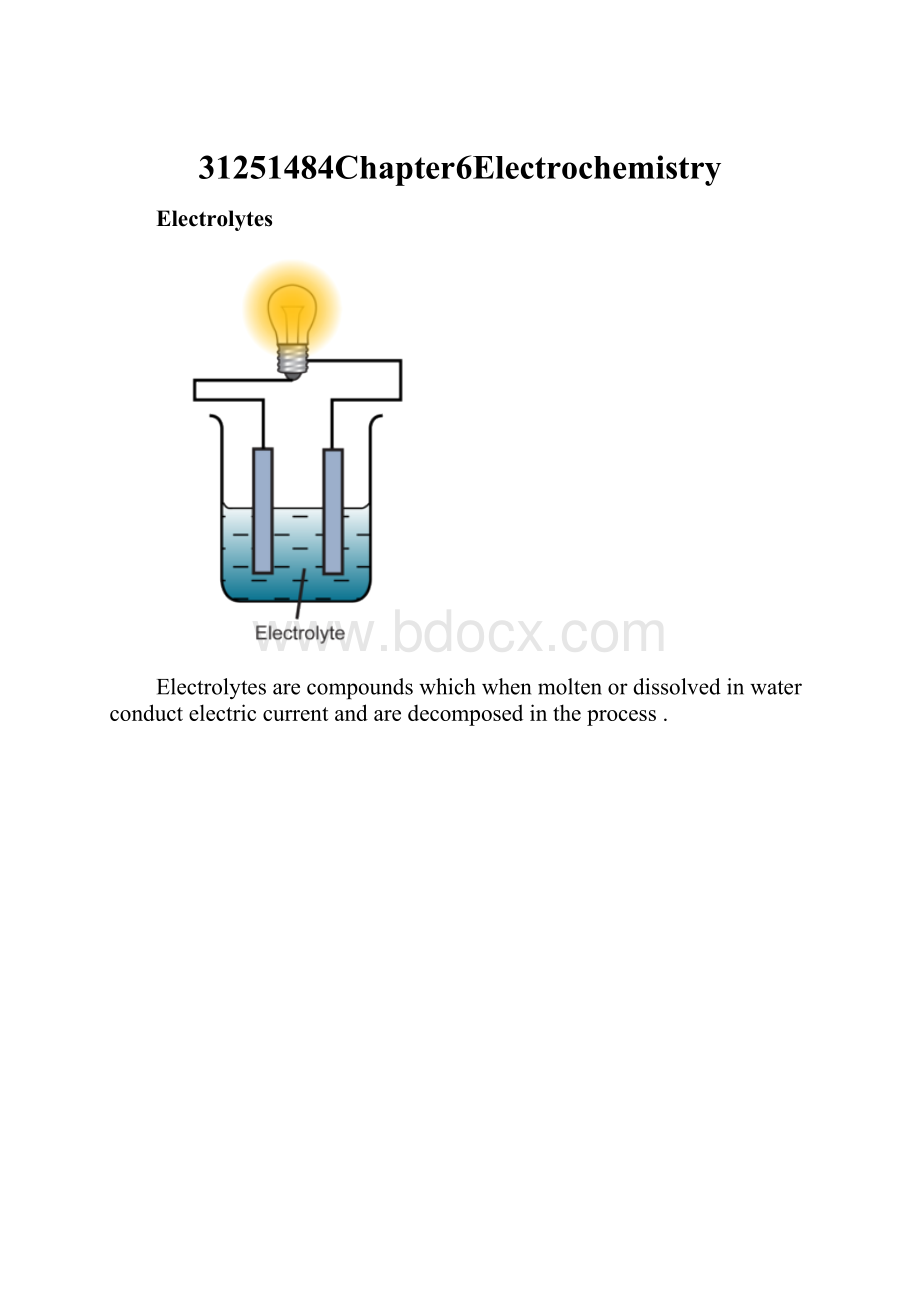
Electrolytes
Electrolytesarecompoundswhichwhenmoltenordissolvedinwaterconductelectriccurrentandaredecomposedintheprocess.
Non-electrolyte
Anon-electrolyteisaliquidwhichdoesnotallowthepassageofelectricity.
MoltenSolution
▪Thisiscomposedoflead(II)ions, Pb2+ ,andbromideions,Br-.ItschemicalformulaisthereforePbBr2.
▪AsuitableapparatuswhichcouldbeusedtocarryoutthiselectrolysisisshowninFigureabove.
▪Thebulbhelpstoshowwhenelectricityisflowinginthecircuit,anduntilthelead(II)bromideiscompletelymolten,thebulbdoesnotlightup.Thisconfirmsthatelectrolyteshavetobemoltenfortheionstostarttomovetotheelectrodesandtherebyconductelectricity.
AttheCathode
AttheAnode
Observation
▪Whenelectricityisflowing,asilverydepositofleadmetalformsonthecathode.Infact,asitismolten,itismorelikelytodripoffinamoltenblob.
Observation
▪Whenelectricityisflowing,brownfumesofbrominegasareseenattheanode.
Halfequation
Pb2+ +2e--->Pb
Halfequation
2Br- --->Br2 +e
Explanation
▪Thelead(II)ions,astheyarepositive,movetothenegativecathode,whereeachiongainstwoelectronstoformaleadatom.
▪Anyreactionatacathodeinvolvedisagaininelectrons.Thisiscalledreductionormoreexactly,cathodicreduction.
Explanation
▪Thebromideions,astheyarenegative,movetothepositiveanode,whereeachlosesanelectrontoformabromineatom.
▪Thentwoofthesenewlyformedatomscombinetoformbrominegas.
▪Anyreactionatananodeinvolvesalossofelectrons.
▪Insummary,thelead(II)bromideissplitintoitscomponentelements :
PbBr2 --->Pb+Br2
ElectrolysisofAqueousSulphuricAcid
▪Assulphuricacidisaqueous,itiscomposednotonlyofhydrogenions(H+ )andsulphateions(SO42-),butalsoofhydroxideions(OH-)fromthewater.
H2SO4 +H2O-->2H+ +SO42- +H+ +OH-
▪TheapparatususedtocarryoutthiselectrolysisandcollectthegasesgivenoffisshowninFigure9.8.
▪Whenwehavemorethanonetypeofionmovingtoanelectrode,selectivedischarge(orpreferentialdischarge)takesplace.
▪Thismeansthattheionwhichcanloseorgainelectronswiththegreatesteaseisdischarged,andtheotherions,whicharehardertodischarge,remaininsolution.
▪Withtheelectrolyteaqueoussulphuricacid,migrationofionstotheelectrodesalsooccurs.
AttheCathode
AttheAnode
▪Herewehaveonlyoneion,thehydrogen,H+(aq),andeachiongainsanelectrontobecomeahydrogenatom.
▪Twoofthesenewlyformedatomsthencombinetoformahydrogengasmolecule.
▪Herewehaveachoiceofeithersulphate,SO42-(aq),orhydroxideOH- (aq)ions.
▪Hydroxideiseasiertodischarge,sooxygengasisgivenoffattheanode.
Equation:
2H+ +2e--->H2
Equation:
OH- +4e--->O2 +H2O
Notes
▪Withelectrolysisofaqueoussolutionsofdiluteacidsoralkalis,thevolumeofhydrogengivenoffatthecathodeisroughlytwicethatoftheoxygengasattheanode.
▪Accordingly,theelementsofwaterarelostandastheelectrolysiscontinues,theconcentrationoftheacidoralkaliincreases.
▪Essentially,theelectrolysisofaqueoussulphuricacidistheelectrolysisofwater,withhydrogenandoxygengasbeinggivenoffinaratioof2 :
1.
ExtractionofMetal
▪Theextractionofmetalsfromtheirores,inparticularaluminiumandsodium,isimportantindustrialusesofelectrolysis.
▪Thediagrambelowshowsthemethodsofextractionfordifferentmetals.
▪Wecanseethatthosemetalswhicharelessreactivethancarboninreactivityseriesareextractedfromtheirorebydisplacementreactionusingcarbon.Thiswillbediscussedindetailinchapter3,form5,OxidationandReduction.
▪Copperandmercurycanbeextractedfromtheirorebyburningdirectlyinair.
▪Silver(Ag)andgold(Au)neednoextractionbecausetheyexistaselementinnature.
▪Thosemetalswhicharemorereactivethancarbonareextractedbyelectrolysis.
ExtractionofAluminium
▪Aluminiumisthemostabundantmetalfoundintheearth'scrust.Itmakesupabout8%byweightoftheEarth’ssolidsurface.
▪Itisalsoaveryusefulmetalduetoitslowdensityandabilitytoresistcorrosion.
▪Themainsourceofaluminiumisbauxiteore(AluminiumOxide).
▪Inindustry,aluminiumisextractedbyelectrolysisfrombauxiteore.
AddingCryolite
▪Inelectrolysis,moltenaluminiumoxidemustbeusedtoextractaluminium.Aluminiumoxidedecomposetoformaluminiumandoxideionswhenmelted.
Al2O3 --->2Al3+ +3O2-
▪However,themeltingpointofaluminiumoxideisveryhigh(over2000°C),soanotheraluminiumcompoundcalledcryolite(Na3AIF6)isaddedtolowerdownthemeltingpoint(about980oC).
▪Thediagramaboveshowshowaluminiumisextractedfrommoltenaluminiumoxidebyelectrolysis.
▪Graphiteisusedastheanodeandcathode.
▪Duringelectrolysis,thealuminiumionsareattractedtowardsthegraphitecathode.
▪Theionsisdischargedandbecomemoltenaluminiummetal.
▪Thepartialequationofthisreactionisasfollow:
Al3+ +3e--->Al
▪Attheanode,oxygengaswhichalsohascommercialvalueiscollected.Thepartialequationofthisreactionisasfollow:
2O2- --->O2 +4e
▪Atthetemperatureof980°C,theoxygenburnsthecarbonanode.Thereforetheanodehastobereplacedperiodically.
▪Also,thiscelluseslargequantitiesofelectricity,andthereforeneedscheapsourcesofpower.
Extractionofsodiumchloride
▪Inindustry,sodiumisextractedfrommoltensodiumchloride.Moltensodiumchlorideisputintotheapparatusasshowinginthediagramabove.
▪Whensodiumchlorideismelted,thesodiumandchlorideionsdisassociatetobecomefreelymoveions,asshowninthechemicalequationbelow.
NaCl--->Na+ +Cl-
▪Inthiselectrolyticcell,graphitewasusedasanodewhileironisusedascathode.
▪Thenegativechlorideionsareattractedtotheanodeandthendischargedtoformchlorinegas.
2Cl- --->Cl2 +2e
▪Sincechlorinegasisalsosignificantinindustry,itiscollectedandstored.
▪Incathode,thesodiumionsaredischargedtoformsodiumatom.
Na+ +e--->Na
▪Duetohightemperature,thesodiummetalformedisinmoltenform.
▪Metalsodiumhavelowerdensity.Thereforeitmovesupwardandbeencollected.
PurificationOfCopper
▪Intherefiningorpurificationofcopper,theimpurecopperismadetheanodeandathin,purecopperplateisusedasacathode.
▪Theelectrolyteisusuallyacidifiedcopper(II)sulphatesolution.
▪Whenelectricityflows,thecopperdissolvesfromtheimpureanodeandgoesintosolutionascopperions.
▪Impuritiesinthecopperdonotdissolve,andinsteadfallofftheanodeasanodesludge.Atthecathode,thecopperionsaredepositedaspurecoppermetal.
▪Reactioninanode(impurecopper)
Inanode,thecopperatomsfromtheelectrodeareionisedtoformcopper(II)ions.
Cu--->Cu2+ +2e
▪Reactionincathode(purecopper)
Cu2+Cu--->Cu+2e
Electroplating
Electroplating:
CoatingwithaThinProtectiveLayerofMetal
▪Averycommonuseofelectrolysisistoformathinprotectivecoatingofametalonthesurfaceofanotherwhichislikelytocorrode.
▪Thediagramaboveillustratetheelectroplatingofakeywithcopper.
▪Inthisprocess,weneedtomakethecathodetheobjectforplating(thekey.
▪Theanodeisthenmadeofthemetalwewishtoplatewith(copper),andtheelectrolyteneedstobeasolutionofasaltofthismetal(copper(II)sulphate).
Anode
▪Inanode,thecopperatomsfromtheelectrodeareionisedtoformcopper(II)ions.
Cu--->Cu2+ +2e
Cathode
▪Incathode,thecopperionsaredischargedtoformcopperatomandthendepositonthesurfaceofthekey
Cu2+ --->Cu+2e
CellsandBatteries
▪Adevicewhichconvertschemicalenergyintoelectricalenergyiscalledacellorbattery.Batteryisacollectionofcells.
▪Acellconsistsofapairofdissimilarmetalsinanelectrolyte.
▪Figureaboveshowsanexampleofasimplevoltaiccellconsistofamagnesiumelectrodeandacopperelectrodeimmerseinmagnesiumsulphatesolution.
▪Whenchemicalreactionhappens,themorereactivemetal,magnesium,dissolvesinthemagnesiumsulphatesolutionandbecomemagnesiumions,therebyproducingelectrons,asshowninthehalfequationbelow:
Mg--->Mg2+ +2e
▪Aselectronsareproduced,themagnesiumactsasthenegativeelectrode.
▪Theseelectronsthentraveltothecopperelectrode.
▪Thehydrogenionsaroundthecopperelectrodereceivetheelectronsandaredischargedtoproducebubblesofhydrogengas:
2H+ +2e--->H2
▪Aselectronsaretakenin,thecopperisthepositiveelectrode.
▪Thisproductionandmovementofelectronsiselectricity,soelectricalenergyhasbeengeneratedandthegalvanometerisdeflected.*Overall,thechemicalreactioncanberepresentedbytheionicequation:
Mg+2H+ --->Mg2+ +H2
▪Involtaiccell,thenegativeelectrodeistheanodewhereasthepositiveelectrodeisthecathode,whichistheoppositeoftheelectrolyticcell.