标准电极电势.docx
《标准电极电势.docx》由会员分享,可在线阅读,更多相关《标准电极电势.docx(19页珍藏版)》请在冰豆网上搜索。
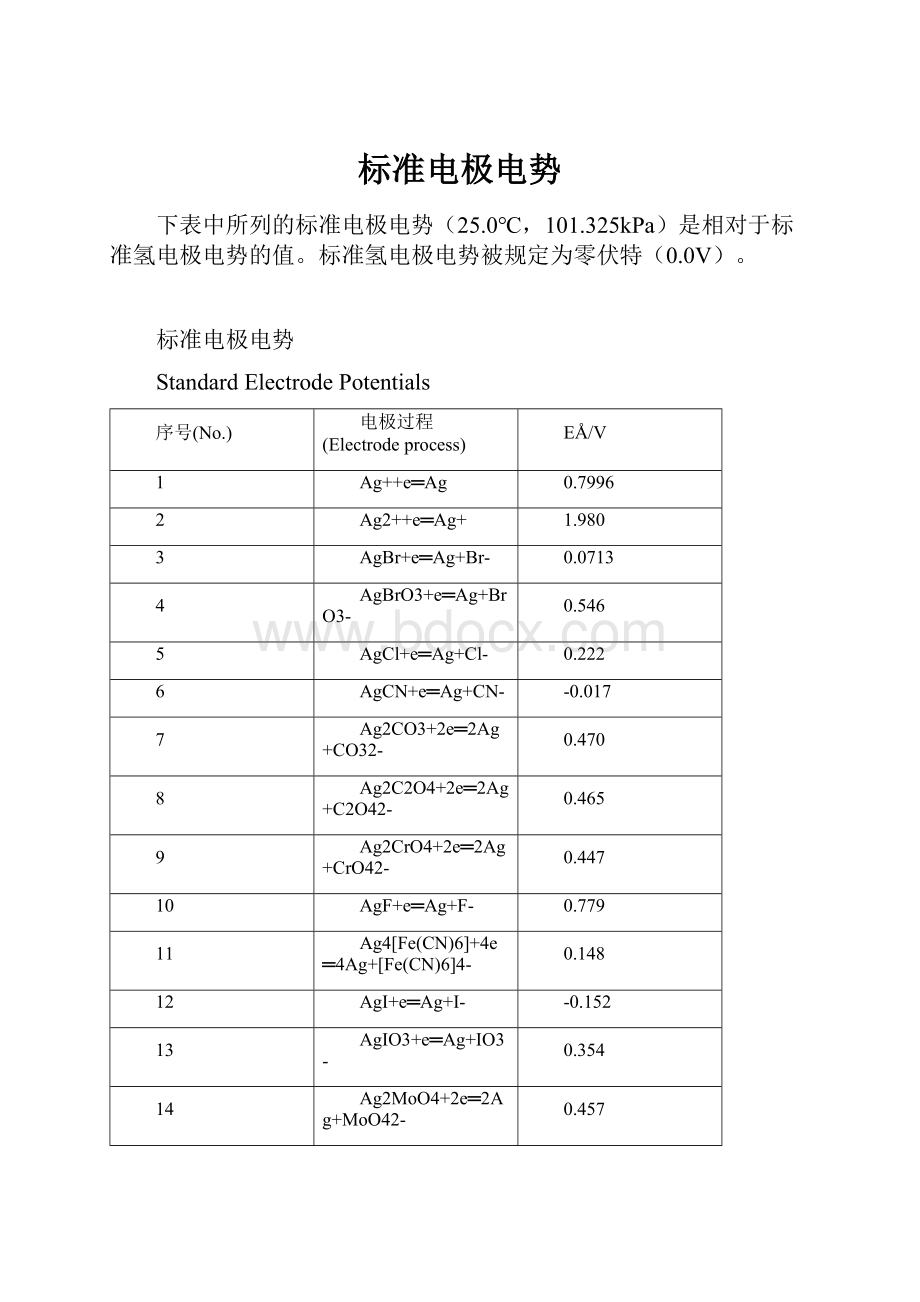
标准电极电势
下表中所列的标准电极电势(25.0℃,101.325kPa)是相对于标准氢电极电势的值。
标准氢电极电势被规定为零伏特(0.0V)。
标准电极电势
StandardElectrodePotentials
序号(No.)
电极过程(Electrodeprocess)
EÅ/V
1
Ag++e═Ag
0.7996
2
Ag2++e═Ag+
1.980
3
AgBr+e═Ag+Br-
0.0713
4
AgBrO3+e═Ag+BrO3-
0.546
5
AgCl+e═Ag+Cl-
0.222
6
AgCN+e═Ag+CN-
-0.017
7
Ag2CO3+2e═2Ag+CO32-
0.470
8
Ag2C2O4+2e═2Ag+C2O42-
0.465
9
Ag2CrO4+2e═2Ag+CrO42-
0.447
10
AgF+e═Ag+F-
0.779
11
Ag4[Fe(CN)6]+4e═4Ag+[Fe(CN)6]4-
0.148
12
AgI+e═Ag+I-
-0.152
13
AgIO3+e═Ag+IO3-
0.354
14
Ag2MoO4+2e═2Ag+MoO42-
0.457
15
[Ag(NH3)2]++e═Ag+2NH3
0.373
16
AgNO2+e═Ag+NO2-
0.564
17
Ag2O+H2O+2e═2Ag+2OH-
0.342
18
2AgO+H2O+2e═Ag2O+2OH-
0.607
19
Ag2S+2e═2Ag+S2-
-0.691
20
Ag2S+2H++2e═2Ag+H2S
-0.0366
21
AgSCN+e═Ag+SCN-
0.0895
22
Ag2SeO4+2e═2Ag+SeO42-
0.363
23
Ag2SO4+2e═2Ag+SO42-
0.654
24
Ag2WO4+2e═2Ag+WO42-
0.466
25
Al3+3e═Al
-1.662
26
AlF63-+3e═Al+6F-
-2.069
27
Al(OH)3+3e═Al+3OH-
-2.31
28
AlO2-+2H2O+3e═Al+4OH-
-2.35
29
Am3++3e═Am
-2.048
30
Am4++e═Am3+
2.60
31
AmO22++4H++3e═Am3++2H2O
1.75
32
As+3H++3e═AsH3
-0.608
33
As+3H2O+3e═AsH3+3OH-
-1.37
34
As2O3+6H++6e═2As+3H2O
0.234
35
HAsO2+3H++3e═As+2H2O
0.248
36
AsO2-+2H2O+3e═As+4OH-
-0.68
37
H3AsO4+2H++2e═HAsO2+2H2O
0.560
38
AsO43-+2H2O+2e═AsO2-+4OH-
-0.71
39
AsS2-+3e═As+2S2-
-0.75
40
AsS43-+2e═AsS2-+2S2-
-0.60
41
Au++e═Au
1.692
42
Au3++3e═Au
1.498
43
Au3++2e═Au+
1.401
44
AuBr2-+e═Au+2Br-
0.959
45
AuBr4-+3e═Au+4Br-
0.854
46
AuCl2-+e═Au+2Cl-
1.15
47
AuCl4-+3e═Au+4Cl-
1.002
48
AuI+e═Au+I-
0.50
49
Au(SCN)4-+3e═Au+4SCN-
0.66
50
Au(OH)3+3H++3e═Au+3H2O
1.45
51
BF4-+3e═B+4F-
-1.04
52
H2BO3-+H2O+3e═B+4OH-
-1.79
53
B(OH)3+7H++8e═BH4-+3H2O
-.0481
54
Ba2++2e═Ba
-2.912
55
Ba(OH)2+2e═Ba+2OH-
-2.99
56
Be2++2e═Be
-1.847
57
Be2O32-+3H2O+4e═2Be+6OH-
-2.63
58
Bi++e═Bi
0.5
59
Bi3++3e═Bi
0.308
60
BiCl4-+3e═Bi+4Cl-
0.16
61
BiOCl+2H++3e═Bi+Cl-+H2O
0.16
62
Bi2O3+3H2O+6e═2Bi+6OH-
-0.46
63
Bi2O4+4H++2e═2BiO++2H2O
1.593
64
Bi2O4+H2O+2e═Bi2O3+2OH-
0.56
65
Br2(水溶液,aq)+2e═2Br-
1.087
66
Br2(液体)+2e═2Br-
1.066
67
BrO-+H2O+2e═Br-+2OH
0.761
68
BrO3-+6H++6e═Br-+3H2O
1.423
69
BrO3-+3H2O+6e═Br-+6OH-
0.61
70
2BrO3-+12H++10e═Br2+6H2O
1.482
71
HBrO+H++2e═Br-+H2O
1.331
72
2HBrO+2H++2e═Br2(水溶液,aq)+2H2O
1.574
73
CH3OH+2H++2e═CH4+H2O
0.59
74
HCHO+2H++2e═CH3OH
0.19
75
CH3COOH+2H++2e═CH3CHO+H2O
-0.12
76
(CN)2+2H++2e═2HCN
0.373
77
(CNS)2+2e═2CNS-
0.77
78
CO2+2H++2e═CO+H2O
-0.12
79
CO2+2H++2e═HCOOH
-0.199
80
Ca2++2e═Ca
-2.868
81
Ca(OH)2+2e═Ca+2OH-
-3.02
82
Cd2++2e═Cd
-0.403
83
Cd2++2e═Cd(Hg)
-0.352
84
Cd(CN)42-+2e═Cd+4CN-
-1.09
85
CdO+H2O+2e═Cd+2OH-
-0.783
86
CdS+2e═Cd+S2-
-1.17
87
CdSO4+2e═Cd+SO42-
-0.246
88
Ce3++3e═Ce
-2.336
89
Ce3++3e═Ce(Hg)
-1.437
90
CeO2+4H++e═Ce3++2H2O
1.4
91
Cl2(气体)+2e═2Cl-
1.358
92
ClO-+H2O+2e═Cl-+2OH-
0.89
93
HClO+H++2e═Cl-+H2O
1.482
94
2HClO+2H++2e═Cl2+2H2O
1.611
95
ClO2-+2H2O+4e═Cl-+4OH-
0.76
96
2ClO3-+12H++10e═Cl2+6H2O
1.47
97
ClO3-+6H++6e═Cl-+3H2O
1.451
98
ClO3-+3H2O+6e═Cl-+6OH-
0.62
99
ClO4-+8H++8e═Cl-+4H2O
1.38
100
2ClO4-+16H++14e═Cl2+8H2O
1.39
101
Cm3++3e═Cm
-2.04
102
Co2++2e═Co
-0.28
103
[Co(NH3)6]3++e═[Co(NH3)6]2+
0.108
104
[Co(NH3)6]2++2e═Co+6NH3
-0.43
105
Co(OH)2+2e═Co+2OH-
-0.73
106
Co(OH)3+e═Co(OH)2+OH-
0.17
107
Cr2++2e═Cr
-0.913
108
Cr3++e═Cr2+
-0.407
109
Cr3++3e═Cr
-0.744
110
[Cr(CN)6]3-+e═[Cr(CN)6]4-
-1.28
111
Cr(OH)3+3e═Cr+3OH-
-1.48
112
Cr2O72-+14H++6e═2Cr3++7H2O
1.232
113
CrO2-+2H2O+3e═Cr+4OH-
-1.2
114
HCrO4-+7H++3e═Cr3++4H2O
1.350
115
CrO42-+4H2O+3e═Cr(OH)3+5OH-
-0.13
116
Cs++e═Cs
-2.92
117
Cu++e═Cu
0.521
118
Cu2++2e═Cu
0.342
119
Cu2++2e═Cu(Hg)
0.345
120
Cu2++Br-+e═CuBr
0.66
121
Cu2++Cl-+e═CuCl
0.57
122
Cu2++I-+e═CuI
0.86
123
Cu2++2CN-+e═[Cu(CN)2]-
1.103
124
CuBr2-+e═Cu+2Br-
0.05
125
CuCl2-+e═Cu+2Cl-
0.19
126
CuI2-+e═Cu+2I-
0.00
127
Cu2O+H2O+2e═2Cu+2OH-
-0.360
128
Cu(OH)2+2e═Cu+2OH-
-0.222
129
2Cu(OH)2+2e═Cu2O+2OH-+H2O
-0.080
130
CuS+2e═Cu+S2-
-0.70
131
CuSCN+e═Cu+SCN-
-0.27
132
Dy2++2e═Dy
-2.2
133
Dy3++3e═Dy
-2.295
134
Er2++2e═Er
-2.0
135
Er3++3e═Er
-2.331
136
Es2++2e═Es
-2.23
137
Es3++3e═Es
-1.91
138
Eu2++2e═Eu
-2.812
139
Eu3++3e═Eu
-1.991
140
F2+2H++2e═2HF
3.053
141
F2O+2H++4e═H2O+2F-
2.153
142
Fe2++2e═Fe
-0.447
143
Fe3++3e═Fe
-0.037
144
[Fe(CN)6]3-+e═[Fe(CN)6]4-
0.358
145
[Fe(CN)6]4-+2e═Fe+6CN-
-1.5
146
FeF63-+e═Fe2++6F-
0.4
147
Fe(OH)2+2e═Fe+2OH-
-0.877
148
Fe(OH)3+e═Fe(OH)2+OH-
-0.56
149
Fe3O4+8H++2e═3Fe2++4H2O
1.23
150
Fm3++3e═Fm
-1.89
151
Fr++e═Fr
-2.9
152
Ga3++3e═Ga
-0.549
153
H2GaO3-+H2O+3e═Ga+4OH-
-1.29
154
Gd3++3e═Gd
-2.279
155
Ge2++2e═Ge
0.24
156
Ge4++2e═Ge2+
0.0
157
GeO2+2H++2e═GeO(棕色)+H2O
-0.118
158
GeO2+2H++2e═GeO(黄色)+H2O
-0.273
159
H2GeO3+4H++4e═Ge+3H2O
-0.182
160
2H++2e═H2
0.0000
161
H2+2e═2H-
-2.25
162
2H2O+2e═H2+2OH-
-0.8277
163
Hf4++4e═Hf
-1.55
164
Hg2++2e═Hg
0.851
165
Hg22++2e═2Hg
0.797
166
2Hg2++2e═Hg22+
0.920
167
Hg2Br2+2e═2Hg+2Br-
0.1392
168
HgBr42-+2e═Hg+4Br-
0.21
169
Hg2Cl2+2e═2Hg+2Cl-
0.2681
170
2HgCl2+2e═Hg2Cl2+2Cl-
0.63
171
Hg2CrO4+2e═2Hg+CrO42-
0.54
172
Hg2I2+2e═2Hg+2I-
-0.0405
173
Hg2O+H2O+2e═2Hg+2OH-
0.123
174
HgO+H2O+2e═Hg+2OH-
0.0977
175
HgS(红色)+2e═Hg+S2-
-0.70
176
HgS(黑色)+2e═Hg+S2-
-0.67
177
Hg2(SCN)2+2e═2Hg+2SCN-
0.22
178
Hg2SO4+2e═2Hg+SO42-
0.613
179
Ho2++2e═Ho
-2.1
180
Ho3++3e═Ho
-2.33
181
I2+2e═2I-
0.5355
182
I3-+2e═3I-
0.536
183
2IBr+2e═I2+2Br-
1.02
184
ICN+2e═I-+CN-
0.30
185
2HIO+2H++2e═I2+2H2O
1.439
186
HIO+H++2e═I-+H2O
0.987
187
IO-+H2O+2e═I-+2OH-
0.485
188
2IO3-+12H++10e═I2+6H2O
1.195
189
IO3-+6H++6e═I-+3H2O
1.085
190
IO3-+2H2O+4e═IO-+4OH-
0.15
191
IO3-+3H2O+6e═I-+6OH-
0.26
192
2IO3-+6H2O+10e═I2+12OH-
0.21
193
H5IO6+H++2e═IO3-+3H2O
1.601
194
In++e═In
-0.14
195
In3++3e═In
-0.338
196
In(OH)3+3e═In+3OH-
-0.99
197
Ir3++3e═Ir
1.156
198
IrBr62-+e═IrBr63-
0.99
199
IrCl62-+e═IrCl63-
0.867
200
K++e═K
-2.931
201
La3++3e═La
-2.379
202
La(OH)3+3e═La+3OH-
-2.90
203
Li++e═Li
-3.040
204
Lr3++3e═Lr
-1.96
205
Lu3++3e═Lu
-2.28
206
Md2++2e═Md
-2.40
207
Md3++3e═Md
-1.65
208
Mg2++2e═Mg
-2.372
209
Mg(OH)2+2e═Mg+2OH-
-2.690
210
Mn2++2e═Mn
-1.185
211
Mn3++3e═Mn
1.542
212
MnO2+4H++2e═Mn2++2H2O
1.224
213
MnO4-+4H++3e═MnO2+2H2O
1.679
214
MnO4-+8H++5e═Mn2++4H2O
1.507
215
MnO4-+2H2O+3e═MnO2+4OH-
0.595
216
Mn(OH)2+2e═Mn+2OH-
-1.56
217
Mo3++3e═Mo
-0.200
218
MoO42-+4H2O+6e═Mo+8OH-
-1.05
219
N2+2H2O+6H++6e═2NH4OH
0.092
220
2NH3OH++H++2e═N2H5++2H2O
1.42
221
2NO+H2O+2e═N2O+2OH-
0.76
222
2HNO2+4H++4e═N2O+3H2O
1.297
223
NO3-+3H++2e═HNO2+H2O
0.934
224
NO3-+H2O+2e═NO2-+2OH-
0.01
225
2NO3-+2H2O+2e═N2O4+4OH-
-0.85
226
Na++e═Na
-2.713
227
Nb3++3e═Nb
-1.099
228
NbO2+4H++4e═Nb+2H2O
-0.690
229
Nb2O5+10H++10e═2Nb+5H2O
-0.644
230
Nd2++2e═Nd
-2.1
231
Nd3++3e═Nd
-2.323
232
Ni2++2e═Ni
-0.257
233
NiCO3+2e═Ni+CO32-
-0.45
234
Ni(OH)2+2e═Ni+2OH-
-0.72
235
NiO2+4H++2e═Ni2++2H2O
1.678
236
No2++2e═No
-2.50
237
No3++3e═No
-1.20
238
Np3++3e═Np
-1.856
239
NpO2+H2O+H++e═Np(OH)3
-0.962
240
O2+4H++4e═2H2O
1.229
241
O2+2H2O+4e═4OH-
0.401
242
O3+H2O+2e═O2+2OH-
1.24
243
Os2++2e═Os
0.85
244
OsCl63-+e═Os2++6Cl-
0.4
245
OsO2+2H2O+4e═Os+4OH-
-0.15
246
OsO4+8H++8e═Os+4H2O
0.838
247
OsO4+4H++4e═OsO2+2H2O
1.02
248
P+3H2O+3e═PH3(g)+3OH-
-0.87
249
H2PO2-+e═P+2OH-
-1.82
250
H3PO3+2H++2e═H3PO2+H2O
-0.499
251
H3PO3+3H++3e═P+3H2O
-0.454
252
H3PO4+2H++2e═H3PO3+H2O-
-0.276
253
PO43-+2H2O+2e═HPO32-+3OH-
-1.05
254
Pa3++3e═Pa
-1.34
255
Pa4++4e═Pa
-1.49
256
Pb2++2e═Pb
-0.126
257
Pb2++2e═Pb(Hg)
-0.121
258
PbBr2+2e═Pb+2Br-
-0.284
259
PbCl2+2e═Pb+2Cl-
-0.268
260
PbCO3+2e═Pb+CO32-
-0.506
261
PbF2+2e═Pb+2F-
-0.344
262
PbI2+2e═Pb+2I-
-0.365
263
PbO+H2O+2e═Pb+2OH-
-0.580
264
PbO+4H++2e═Pb+H2O
0.25
265
PbO2+4H++2e═Pb2+2H2O
1.455
266
HPbO2-+H2O+2e═Pb+3OH-
-0.537
267
PbO2+SO42-+4H++2e═PbSO4+2H2O
1.691
268
PbSO4+2e═Pb+SO42-
-0.359
269
Pd2++2e═Pd
0.915
270
PdBr42-+2e═Pd+4Br-
0.6
271
PdO2+H2O+2e═PdO+2OH-
0.73
272
Pd(OH)2+2e═Pd+2OH-
0.07
273
Pm2++2e═Pm
-2.20
274
Pm3++3e═Pm
-2.30
275
Po4++4e═Po
0.76
276
Pr2++2e═Pr
-2.0
277
Pr3++3e═Pr
-2.353
278
Pt2++2e═Pt
1.18
279
[PtCl6]2-+2e═[PtCl4]2-+2Cl-
0.68
280
Pt(OH)2+2e═Pt+2OH-
0.14
281
PtO2+4H++4e═Pt+2H2O
1.00
282
PtS+2e═Pt+S2-
-0.83
283
Pu3++3e═Pu
-2.031
284
Pu5++e═Pu4+
1.099
285
Ra2++2e═Ra
-2.8
286
Rb++e═Rb
-2.98
287
Re3++3e═Re
0.300
288
ReO2+4H++4e═Re+2H2O
0.251
289
ReO4-+4H++3e═ReO2+2H2O
0.510
290
ReO4-+4H2O+7e═Re+8OH-
-0.584
291
Rh2++2e═Rh
0.600
292
Rh3++3e═Rh
0.758
293
Ru2++2e═Ru
0.455
294
RuO2+4H++2e═Ru2++2H2O
1.120
295
RuO4+6H++4e═Ru(OH)22++2H2O
1.40
296
S+2e═S2-
-0.476
297
S+2H++2e═H2S(水溶液,aq)
0.142
298
S2O62-+4H++2e═2H2SO3
0.564
299
2SO32-+3H2O+4e═S2O32-+6OH-
-0.571
300
2SO32-+2H2O+2e═S2O42-+4OH-
-1.12
301
SO42-+H2O+2e═SO32-+2OH-
-0.93
302
Sb+3H++3e═SbH3
-0.510
303
Sb2O3+6H++6e═2Sb+3H2O
0.152
304
Sb2O5+6H++4e═2SbO++3H2O
0.581
305
SbO3-+H2O+2e═SbO2-+2OH-
-0.59
306
Sc3++3e═Sc
-2.077
307
Sc(OH)3+3e═Sc+3OH-
-2.6
308
Se+2e═Se2-
-0.924
309
Se+2H++2e═H2Se(水溶液,aq)
-0.399
310
H2SeO3+4H++4e═Se+3H2O
-0.74
311
SeO32-+3H2O+4e═Se+6OH-
-0.366
312
SeO42-+H2O+2e═SeO32-+2OH-
0.05
313
Si+4H++4e═SiH4(气体)
0.102
314
Si+4H2O+4e═SiH4+4OH-
-0.73
315
SiF62-+4e═Si+6F-
-1.24
316
SiO2+4H++4e═Si+2H2O
-0.857
317
SiO32-+3H2O+4e═Si+6OH-
-1.697
318
Sm2++2e═Sm
-2.68
319
Sm3++3e═Sm
-2.304
320
Sn2++2e═Sn
-0.138
321
Sn4++2e═Sn2+
0.151
322
SnCl42-+2e═Sn+4Cl-(1mol/LHCl)
-0.19
323
SnF62-+4e═Sn+6F-
-0.25
324
Sn(OH)3-+3H++2e═Sn2++3H2O
0.142
325
SnO2+4H++4e═Sn+2H2O
-0.117